Regulation of coenzyme A levels by degradation: the 'Ins and Outs'
- PMID: 32234503
- PMCID: PMC7234920
- DOI: 10.1016/j.plipres.2020.101028
Regulation of coenzyme A levels by degradation: the 'Ins and Outs'
Abstract
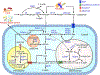
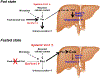
Coenzyme A (CoA) is the predominant acyl carrier in mammalian cells and a cofactor that plays a key role in energy and lipid metabolism. CoA and its thioesters (acyl-CoAs) regulate a multitude of metabolic processes at different levels: as substrates, allosteric modulators, and via post-translational modification of histones and other non-histone proteins. Evidence is emerging that synthesis and degradation of CoA are regulated in a manner that enables metabolic flexibility in different subcellular compartments. Degradation of CoA occurs through distinct intra- and extracellular pathways that rely on the activity of specific hydrolases. The pantetheinase enzymes specifically hydrolyze pantetheine to cysteamine and pantothenate, the last step in the extracellular degradation pathway for CoA. This reaction releases pantothenate in the bloodstream, making this CoA precursor available for cellular uptake and de novo CoA synthesis. Intracellular degradation of CoA depends on specific mitochondrial and peroxisomal Nudix hydrolases. These enzymes are also active against a subset of acyl-CoAs and play a key role in the regulation of subcellular (acyl-)CoA pools and CoA-dependent metabolic reactions. The evidence currently available indicates that the extracellular and intracellular (acyl-)CoA degradation pathways are regulated in a coordinated and opposite manner by the nutritional state and maximize the changes in the total intracellular CoA levels that support the metabolic switch between fed and fasted states in organs like the liver. The objective of this review is to update the contribution of these pathways to the regulation of metabolism, physiology and pathology and to highlight the many questions that remain open.
Keywords: Coenzyme A; Metabolic regulation; Nudix hydrolase; Pantetheinases; Pantothenate/organelles.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures

References
-
- Depeint F, Bruce WR, Shangari N, Mehta R, O’Brien PJ. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact 2006;163:94–112. https://10.1016/j.cbi.2006.04.014. - DOI - PubMed
-
- Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol 2014;15:536–50. https://10.1038/nrm3841. - DOI - PubMed
-
- Hirschey MD, Zhao Y. Metabolic Regulation by Lysine Malonylation, Succinylation, and Glutarylation. Mol Cell Proteomics 2015;14:2308–15. https://10.1074/mcp.R114.046664. - DOI - PMC - PubMed
-
- Resh MD. Fatty acylation of proteins: The long and the short of it. Prog Lipid Res 2016;63:120–31. https://10.1016/j.plipres.2016.05.002. - DOI - PMC - PubMed
-
- Daniotti JL, Pedro MP, Valdez Taubas J. The role of S-acylation in protein trafficking. Traffic 2017. https://10.1111/tra.12510. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

