4-1BB costimulation promotes CAR T cell survival through noncanonical NF-κB signaling
- PMID: 32234960
- PMCID: PMC7883633
- DOI: 10.1126/scisignal.aay8248
4-1BB costimulation promotes CAR T cell survival through noncanonical NF-κB signaling
Abstract
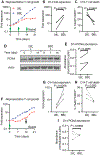
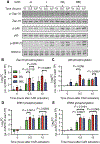
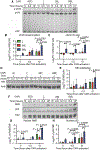
Clinical response to chimeric antigen receptor (CAR) T cell therapy is correlated with CAR T cell persistence, especially for CAR T cells that target CD19+ hematologic malignancies. 4-1BB-costimulated CAR (BBζ) T cells exhibit longer persistence after adoptive transfer than do CD28-costimulated CAR (28ζ) T cells. 4-1BB signaling improves T cell persistence even in the context of 28ζ CAR activation, which indicates distinct prosurvival signals mediated by the 4-1BB cytoplasmic domain. To specifically study signal transduction by CARs, we developed a cell-free, ligand-based activation and ex vivo culture system for CD19-specific CAR T cells. We observed greater ex vivo survival and subsequent expansion of BBζ CAR T cells when compared to 28ζ CAR T cells. We showed that only BBζ CARs activated noncanonical nuclear factor κB (ncNF-κB) signaling in T cells basally and that the anti-CD19 BBζ CAR further enhanced ncNF-κB signaling after ligand engagement. Reducing ncNF-κB signaling reduced the expansion and survival of anti-CD19 BBζ T cells and was associated with a substantial increase in the abundance of the most pro-apoptotic isoforms of Bim. Although our findings do not exclude the importance of other signaling differences between BBζ and 28ζ CARs, they demonstrate the necessary and nonredundant role of ncNF-κB signaling in promoting the survival of BBζ CAR T cells, which likely underlies the engraftment persistence observed with this CAR design.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



References
-
- Feins S, Kong W, Williams EF, Milone MC, Fraietta JA, An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am. J. Hematol 94, S3–S9 (2019). - PubMed
-
- Mueller KT, Waldron E, Grupp SA, Levine JE, Laetsch TW, Pulsipher MA, Boyer MW, August KJ, Hamilton J, Awasthi R, Stein AM, Sickert D, Chakraborty A, Levine BL, June CH, Tomassian L, Shah SS, Leung M, Taran T, Wood PA, Maude SL, Clinical pharmacology of tisagenlecleucel in B-cell acute lymphoblastic leukemia. Clin. Cancer Res 24, 6175–6184 (2018). - PMC - PubMed
-
- Orlando EJ, Han X, Tribouley C, Wood PA, Leary RJ, Riester M, Levine JE, Qayed M, Grupp SA, Boyer M, De Moerloose B, Nemecek ER, Bittencourt H, Hiramatsu H, Buechner J, Davies SM, Verneris MR, Nguyen K, Brogdon JL, Bitter H, Morrissey M, Pierog P, Pantano S, Engelman JA, Winckler W, Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med 24, 1504–1506 (2018). - PubMed
-
- Mueller KT, Maude SL, Porter DL, Frey N, Wood P, Han X, Waldron E, Chakraborty A, Awasthi R, Levine BL, Melenhorst JJ, Grupp SA, June CH, Lacey SF, Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood 130, 2317–2325 (2017). - PMC - PubMed
-
- Turtle CJ, Hanafi L-A, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG, CD19 CAR-T cells of defined CD4+: CD8+composition in adult B cell ALL patients. J. Clin. Invest 126, 2123–2138 (2016). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

