Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP)
- PMID: 32234967
- PMCID: PMC7279201
- DOI: 10.1136/jnnp-2019-322326
Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP)
Abstract
Objective: The Tysabri Observational Programme (TOP), which began >10 years ago, is an open-label, multinational, prospective observational study evaluating the long-term safety and effectiveness of natalizumab in relapsing-remitting multiple sclerosis patients.
Methods: These data provide a 10-year interim analysis of safety and effectiveness in TOP. Annualised relapse rates (ARRs) and disability progression/improvement were analysed using the Poisson model and the Kaplan-Meier method, respectively. Analyses included patients on natalizumab and those who discontinued natalizumab but remained in TOP.
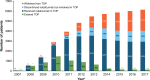
Results: As of November 2017, TOP included 6148 patients. Overall, 829 patients (13.5%) experienced ≥1 serious adverse event (SAE), with infection the most common (4.1%). Fifty-three patients (0.9%) had confirmed progressive multifocal leukoencephalopathy. SAE data were consistent with natalizumab's known safety profile; no new safety signals were identified. A total of 3210 patients (52.2%) discontinued natalizumab; 2117 (34.4%) withdrew from TOP. Median time on natalizumab was 3.3 (range 0-11.6) years; median follow-up time was 5.2 (range 0-10.8) years. The on-natalizumab ARR was 0.15, a 92.5% reduction from the year before initiation. Ten-year cumulative probabilities of disability worsening and improvement were 27.8% and 33.1%, respectively. On-natalizumab ARRs were similar between patients who discontinued or remained on natalizumab, suggesting limited attrition bias.
Conclusions: Since the TOP 5-year interim analysis (December 2012), cohort size (6148 vs 4821), median exposure (3.3 vs 1.8 years) and median follow-up time (62 vs 26 months) have increased. This 10-year interim analysis further supports the robust real-world effectiveness and well-established safety profile of natalizumab.
Trial registration number: NCT00493298.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: HB has received compensation for steering committee, advisory board and consultancy fees from Biogen, Merck, Roche, Novartis, Oxford Pharmagenesis and Teva and research support from Biogen, Merck, Novartis, MS Research Australia, NHMRC Australia and the UK MS Trust. LK’s institution (University Hospital Basel) has received the following in the last 3 years and used exclusively for research support at the department: steering committee, advisory board and consultancy fees from Actelion, Alkermes, Almirall, Bayer, Biogen, Celgene/Receptos, df-mp, Excemed, GeNeuro SA, Genzyme, Japan Tobacco, Merck, Minoryx, Mitsubishi Pharma, Novartis, Roche, Sanofi, Santhera, Teva and Vianex, as well as royalties for Neurostatus-UHB products; the Research of the MS Centre in Basel has been supported by grants from Bayer, Biogen, Novartis, the European Union, the Roche Research Foundations, the Swiss MS Society and the Swiss National Research Foundation. HW has received honoraria from AbbVie, Actelion, Alexion, Biogen, Cognomed, Evgen, F. Hoffmann-La Roche, MedDay, Merck Serono, Novartis, Roche Pharma AG, Sanofi Genzyme and Teva and research support from Biogen, GlaxoSmithKline GmbH, Roche Pharma AG and Sanofi Genzyme. MT has received compensation for consulting from Biogen, Merck Serono and Novartis; speaker honoraria from Biogen, Merck Serono, Novartis, Sanofi and Teva; and research grants from Biogen, Merck Serono and Novartis. TS has received honoraria for consultancy and funding for travel from Biogen and Novartis. IC, NC, P-RH and SL are employees of and may hold stock and/or stock options in Biogen. RK and SJ were employees of Biogen at the time of these analyses and may hold stock and/or stock options in Biogen.
Figures


References
-
- Tysabri (natalizumab) [summary of product characteristics] 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
