Efficiency of Differently Processed Membranes Based on Cellulose as Cationic Dye Adsorbents
- PMID: 32235489
- PMCID: PMC7221949
- DOI: 10.3390/nano10040642
Efficiency of Differently Processed Membranes Based on Cellulose as Cationic Dye Adsorbents
Abstract
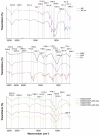
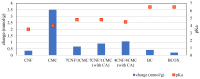
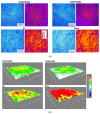
In order to minimize the pollution caused by the reuse of textile dyes, technologies and materials have been developed that purify waste water in an efficient and cost-effective manner before it is discharged into a water body. In this context, the presented research investigates the potential of two types of fully cellulose-based membranes as adsorbents for cationic dyes used in the textile industry. The first type combines cellulose nanofibrils (CNFs) and carboxymethylated cellulose (CMC) using the solvent casting process and an esterification coupling reaction, while the second type uses commercial bacterial cellulose (BC) in a native and sodium periodate-treated form (BCox). The corresponding membranes were comprehensively evaluated by means of Fourier Transform Infrared (FTIR) Spectroscopy. Results confirm the esterification process within the CNF/CMC membranes, as well as BC oxidation after periodate treatment, as shown by bands at 1726.2 cm-1 and 895 cm-1, respectively. The Potentiometric Titration shows the highest total negative charge of 1.07 mmol/g for 4CNF/4CMC, which is assigned to the presence of COO- within CMC polymers, and lowest (0.21 mmol/g) for BCox. The Contact Angle Goniometry data confirm the hydrophilicity of all membranes, and the angle increased from 0 ° (in pure BC) to 34.5 ° in CMC-rich and to 31.4 ° in BCox membranes due to the presence of CH2COO- and CHO groups, respectively. Confocal Fluorescent Microscopy (CFM) demonstrated the highest µ-roughness in 4CNF/4CMC, while Scanning Electron Microscopy (SEM) depicted diverse morphological features between the membranes, from ultrafine nanofiber networks (in BC and BCox) to larger fiber bundles connected within the polymer phase in CNF/CMC membranes. The adsorption experiment followed by UV-VIS spectroscopy, showed ~100% dye removal efficiency in both CNF/CMC-based membranes, while BC and BCox adsorbed only 24.3% and 23.6%, respectively, when anthraquinone dye was used. Azo dye was only adsorbed with an efficiency of 7-9% on CMC/CNF-based membranes, compared with 5.57% on BC and 7.33% on BCox membranes. The adsorption efficiency at equilibrium was highest for BC (1228 mg/g) and lowest for 7CNF/1CMC (419.24 mg/g) during anthraquinone dye adsorption. In the case of azo dye, the BCox was most effective, with 445.7 mg/g. Applicability of a pseudo second-order model was confirmed for both dyes and all membranes, except for BCox in combination with azo dye, showing the fastest adsorption rate in the case of the 7CNF/1CMC membrane.
Keywords: adsorption efficiency; bacterial cellulose; cationic dyes; cellulose membrane; cellulose nanofibrils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
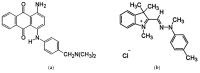







References
-
- Thamaraiselvan C., Noel M. Membrane processes for dye wastewater treatment: Recent progress in fouling control. Crit. Rev. Environ. Sci. Technol. 2015;45:1007–1040. doi: 10.1080/10643389.2014.900242. - DOI
-
- Castañeda-DÍaz J., Pavón-Silva T., Gutiérrez-Segura E., Colín-Cruz A. Electrocoagulation-Adsorption to Remove Anionic and Cationic Dyes from Aqueous Solution by PV-Energy. J. Chem. 2017;2017 doi: 10.1155/2017/5184590. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

