The Production of Listeriolysin O and Subsequent Intracellular Infections by Listeria monocytogenes Are Regulated by Exogenous Short Chain Fatty Acid Mixtures
- PMID: 32235519
- PMCID: PMC7232371
- DOI: 10.3390/toxins12040218
The Production of Listeriolysin O and Subsequent Intracellular Infections by Listeria monocytogenes Are Regulated by Exogenous Short Chain Fatty Acid Mixtures
Abstract
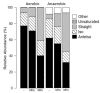
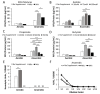
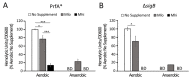
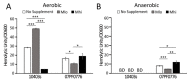
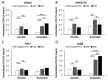
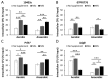
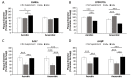
Listeria monocytogenes is a foodborne pathogen capable of secreting listeriolysin O (LLO), a pore-forming toxin encoded by the hly gene. While the functions of LLO have been studied extensively, how the production of LLO is modulated by the intestinal environment, devoid of oxygen and enriched in short chain fatty acids (SCFAs), is not completely understood. Using L. monocytogenes strain 10403s, we found that hly transcription was moderately decreased by aerobic SCFA exposures but significantly increased by anaerobic SCFA exposures. Moreover, aerobic, but not anaerobic, exposure to low levels of SCFAs resulted in a significantly higher LLO activity. These results demonstrated that transcriptional and post-transcriptional regulations of LLO production were separately modulated by SCFAs and were responsive to oxygen levels. Examining isogenic mutants revealed that PrfA and SigB play a role in regulating LLO production in response to SCFAs. Effects of SCFAs were also present in the cardiotropic strain 07PF0776 but distinctly different from those in strain 10403s. For both strains, prior exposures to SCFAs altered intracellular infections in Caco-2 and RAW264.7 cells and the plaque sizes in L fibroblasts, a result confirming the ability of L. monocytogenes to adapt to SCFAs in ways that impact its subsequent infection outcomes.
Keywords: LLO; Listeria monocytogenes; aerobic; anaerobic; hly; short chain fatty acids.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Similar articles
-
Spontaneous Loss of Virulence in Natural Populations of Listeria monocytogenes.Infect Immun. 2017 Oct 18;85(11):e00541-17. doi: 10.1128/IAI.00541-17. Print 2017 Nov. Infect Immun. 2017. PMID: 28827366 Free PMC article.
-
Relative Roles of Listeriolysin O, InlA, and InlB in Listeria monocytogenes Uptake by Host Cells.Infect Immun. 2018 Sep 21;86(10):e00555-18. doi: 10.1128/IAI.00555-18. Print 2018 Oct. Infect Immun. 2018. PMID: 30061379 Free PMC article.
-
The 5' untranslated region-mediated enhancement of intracellular listeriolysin O production is required for Listeria monocytogenes pathogenicity.Mol Microbiol. 2005 Sep;57(5):1460-73. doi: 10.1111/j.1365-2958.2005.04780.x. Mol Microbiol. 2005. PMID: 16102013
-
Listeriolysin O: a phagosome-specific lysin.Microbes Infect. 2007 Aug;9(10):1176-87. doi: 10.1016/j.micinf.2007.05.005. Epub 2007 May 7. Microbes Infect. 2007. PMID: 17720603 Review.
-
Listeriolysin O: A phagosome-specific cytolysin revisited.Cell Microbiol. 2019 Mar;21(3):e12988. doi: 10.1111/cmi.12988. Epub 2019 Jan 15. Cell Microbiol. 2019. PMID: 30511471 Free PMC article. Review.
Cited by
-
The PrfA regulon of Listeria monocytogenes is induced by growth in low-oxygen microaerophilic conditions.Microbiology (Reading). 2024 Nov;170(11):001516. doi: 10.1099/mic.0.001516. Microbiology (Reading). 2024. PMID: 39560979 Free PMC article.
References
-
- USDA Economic Research Service—Cost Estimates of Foodborne Illnesses. [(accessed on 28 March 2015)]; Available online: http://www.ers.usda.gov/data-products/cost-estimates-of-foodborne-illnes....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

