Formation of Size and Density Controlled Nanostructures by Galvanic Displacement
- PMID: 32235596
- PMCID: PMC7221692
- DOI: 10.3390/nano10040644
Formation of Size and Density Controlled Nanostructures by Galvanic Displacement
Abstract
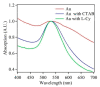
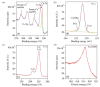
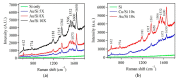
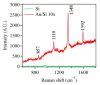
Gold (Au) and copper (Cu)-based nanostructures are of great interest due to their applicability in various areas including catalysis, sensing and optoelectronics. Nanostructures synthesized by the galvanic displacement method often lead to non-uniform density and poor size distribution. Here, density and size-controlled synthesis of Au and Cu-based nanostructures was made possible by galvanic displacement with limited exposure to hydrofluoric (HF) acid and the use of surfactants like L-cysteine (L-Cys) and cetyltrimethylammonium bromide (CTAB). An approach involving cyclic exposure to HF acid regulated the nanostructure density. Further, the use of surfactants generated monodisperse nanoparticles in the initial stages of the deposition with increased density. The characterization of Au and Cu-based nanostructures was performed by scanning electron microscopy, atomic force microscopy, UV-Visible spectroscopy, X-ray photoelectron spectroscopy, Raman spectroscopy and X-ray diffraction. The surface enhanced Raman spectroscopic measurements demonstrated an increase in the Raman intensity by two to three orders of magnitude for analyte molecules like Rhodamine 6G dye and paraoxon.
Keywords: gold; monodisperse; nanostructures; sensing; surfactant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Carraro C., Maboudian R., Magagnin L. Metallization and nanostructuring of semiconductor surfaces by galvanic displacement processes. Surf. Sci. Rep. 2007;62:499–525. doi: 10.1016/j.surfrep.2007.08.002. - DOI
-
- Ali H.O., Christie I.R.A. A review of electroless gold deposition processes. Gold Bull. 1984;17:118–127. doi: 10.1007/BF03214674. - DOI
-
- Darosa C.I., Iglesia E., Maboudian R. Dynamics of Copper Deposition onto Silicon by Galvanic Displacement. J. Electrochem. Soc. 2008;155:6. doi: 10.1149/1.2829907. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

