Behavior and Mechanism of Cesium Biosorption from Aqueous Solution by Living Synechococcus PCC7002
- PMID: 32235603
- PMCID: PMC7232235
- DOI: 10.3390/microorganisms8040491
Behavior and Mechanism of Cesium Biosorption from Aqueous Solution by Living Synechococcus PCC7002
Abstract
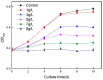
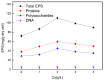
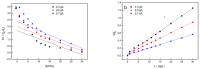
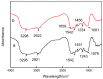
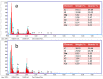
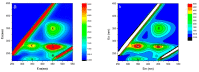
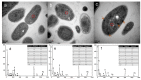
Many efforts have focused on the adsorption of metals from contaminated water by microbes. Synechococcus PCC7002, a major marine cyanobacteria, is widely applied to remove metals from the ocean's photic zone. However, its ability to adsorb cesium (Cs) nuclides has received little attention. In this study, the biosorption behavior of Cs(I) from ultrapure distilled water by living Synechococcus PCC7002 was investigated based on kinetic and isotherm studies, and the biosorption mechanism was characterized by Fourier-transform infrared spectroscopy, Raman spectroscopy, scanning electron microscopy, transmission electron microscopy, energy-dispersive X-ray spectrometry, and three-dimensional excitation emission matrix fluorescence spectroscopy. Synechococcus PCC7002 showed extremely high tolerance to Cs ions and its minimal inhibitory concentration was 8.6 g/L. Extracellular polymeric substances (EPS) in Synechococcus PCC7002 played a vital role in this tolerance. The biosorption of Cs by Synechococcus PCC7002 conformed to a Freundlich-type isotherm model and pseudo-second-order kinetics. The binding of Cs(I) was primarily attributed to the extracellular proteins in EPS, with the amino, hydroxyl, and phosphate groups on the cell walls contributing to Cs adsorption. The biosorption of Cs involved two mechanisms: Passive adsorption on the cell surface at low Cs concentrations and active intracellular adsorption at high Cs concentrations. The results demonstrate that the behavior and mechanism of Cs adsorption by Synechococcus PCC7002 differ based on the Cs ions concentration.
Keywords: Biosorption; Cs; EPS; Synechococcus PCC7002.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jalali-Rad R., Ghafourian H., Asef Y., Dalir S.T., Sahafipour M.H., Gharanjik B.M. Biosorption of cesium by native and chemically modified biomass of marine algae: Introduce the new biosorbents for biotechnology applications. J. Hazard. Mater. 2004;116:125–134. doi: 10.1016/j.jhazmat.2004.08.022. - DOI - PubMed
-
- Tomioka N., Tanaka K., Uchiyama H., Yagi O., Kokufuta E. Recovery of 137Cs by a bioaccumulation system using Rhodococcus erythropolis CS98. J. Ferment. Bioeng. 1998;85:604–608. doi: 10.1016/S0922-338X(98)80013-5. - DOI
Grants and funding
- 31470230, 51320105006 and 51604308/National Natural Science Foundation of China
- 2017RS3003/Youth Talent Foundation of Hunan Province of China
- 2018JJ2486/Natural Science Foundation of Hunan Province of China
- 2018WK2012/Key Research and Development Projects in Hunan Province
- 2019zzts685/the Fundamental Research Funds for the Central Universities of Central South University
LinkOut - more resources
Full Text Sources

