Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement
- PMID: 32235743
- PMCID: PMC7146355
- DOI: 10.3390/nu12030848
Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement
Abstract
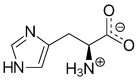
L-histidine (HIS) is an essential amino acid with unique roles in proton buffering, metal ion chelation, scavenging of reactive oxygen and nitrogen species, erythropoiesis, and the histaminergic system. Several HIS-rich proteins (e.g., haemoproteins, HIS-rich glycoproteins, histatins, HIS-rich calcium-binding protein, and filaggrin), HIS-containing dipeptides (particularly carnosine), and methyl- and sulphur-containing derivatives of HIS (3-methylhistidine, 1-methylhistidine, and ergothioneine) have specific functions. The unique chemical properties and physiological functions are the basis of the theoretical rationale to suggest HIS supplementation in a wide range of conditions. Several decades of experience have confirmed the effectiveness of HIS as a component of solutions used for organ preservation and myocardial protection in cardiac surgery. Further studies are needed to elucidate the effects of HIS supplementation on neurological disorders, atopic dermatitis, metabolic syndrome, diabetes, uraemic anaemia, ulcers, inflammatory bowel diseases, malignancies, and muscle performance during strenuous exercise. Signs of toxicity, mutagenic activity, and allergic reactions or peptic ulcers have not been reported, although HIS is a histamine precursor. Of concern should be findings of hepatic enlargement and increases in ammonia and glutamine and of decrease in branched-chain amino acids (valine, leucine, and isoleucine) in blood plasma indicating that HIS supplementation is inappropriate in patients with liver disease.
Keywords: Bretschneider’s solution; HTK solution; ammonia; beta-alanine; branched-chain amino acids; carnosine; glutamine; histidine supplementation.
Conflict of interest statement
The author declares no conflict of interest.
Figures
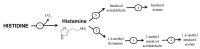

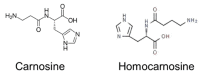

References
-
- Pinals R.S., Harris E.D., Burnett J.B., Gerber D.A. Treatment of rheumatoid arthritis with L-histidine: A randomized, placebo-controlled, double-blind trial. J. Rheumatol. 1977;4:414–419. - PubMed
-
- Matsukura T., Tanaka H. Applicability of zinc complex of L-carnosine for medical use. Biochemistry (Mosc.) 2000;65:817–823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

