High Density Display of an Anti-Angiogenic Peptide on Micelle Surfaces Enhances Their Inhibition of αvβ3 Integrin-Mediated Neovascularization In Vitro
- PMID: 32235802
- PMCID: PMC7153711
- DOI: 10.3390/nano10030581
High Density Display of an Anti-Angiogenic Peptide on Micelle Surfaces Enhances Their Inhibition of αvβ3 Integrin-Mediated Neovascularization In Vitro
Abstract
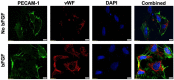
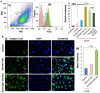
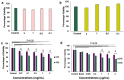
Diabetic retinopathy (DR), Retinopathy of Pre-maturity (ROP), and Age-related Macular Degeneration (AMD) are multifactorial manifestations associated with abnormal growth of blood vessels in the retina. These three diseases account for 5% of the total blindness and vision impairment in the US alone. The current treatment options involve heavily invasive techniques such as frequent intravitreal administration of anti-VEGF (vascular endothelial growth factor) antibodies, which pose serious risks of endophthalmitis, retinal detachment and a multitude of adverse effects stemming from the diverse physiological processes that involve VEGF. To overcome these limitations, this current study utilizes a micellar delivery vehicle (MC) decorated with an anti-angiogenic peptide (aANGP) that inhibits αvβ3 mediated neovascularization using primary endothelial cells (HUVEC). Stable incorporation of the peptide into the micelles (aANGP-MCs) for high valency surface display was achieved with a lipidated peptide construct. After 24 h of treatment, aANGP-MCs showed significantly higher inhibition of proliferation and migration compared to free from aANGP peptide. A tube formation assay clearly demonstrated a dose-dependent angiogenic inhibitory effect of aANGP-MCs with a maximum inhibition at 4 μg/mL, a 1000-fold lower concentration than that required for free from aANGP to display a biological effect. These results demonstrate valency-dependent enhancement in the therapeutic efficacy of a bioactive peptide following conjugation to nanoparticle surfaces and present a possible treatment alternative to anti-VEGF antibody therapy with decreased side effects and more versatile options for controlled delivery.
Keywords: Micelles; PEG-b-PPS; VEGF; anti-angiogenic; integrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Retinopathy of Prematurity|National Eye Institute. [(accessed on 20 December 2019)]; Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseas....
-
- Nowak J.Z. Age-related macular degeneration (AMD): Pathogenesis and therapy. Pharmacol. Rep. PR. 2006;58:353–363. - PubMed
-
- Brey E.M., McIntire L.V. 59—Vascular Assembly in Engineered and Natural Tissues. In: Atala A., Lanza R., Thomson J.A., Nerem R.M., editors. Principles of Regenerative Medicine. Academic Press; San Diego, CA, USA: 2008. pp. 1020–1037.
Grants and funding
LinkOut - more resources
Full Text Sources

