Impaired theta phase coupling underlies frontotemporal dysconnectivity in schizophrenia
- PMID: 32236540
- PMCID: PMC7174039
- DOI: 10.1093/brain/awaa035
Impaired theta phase coupling underlies frontotemporal dysconnectivity in schizophrenia
Abstract
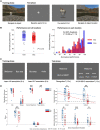
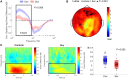
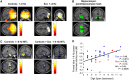
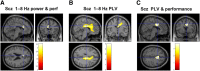
Frontotemporal dysconnectivity is a key pathology in schizophrenia. The specific nature of this dysconnectivity is unknown, but animal models imply dysfunctional theta phase coupling between hippocampus and medial prefrontal cortex (mPFC). We tested this hypothesis by examining neural dynamics in 18 participants with a schizophrenia diagnosis, both medicated and unmedicated; and 26 age, sex and IQ matched control subjects. All participants completed two tasks known to elicit hippocampal-prefrontal theta coupling: a spatial memory task (during magnetoencephalography) and a memory integration task. In addition, an overlapping group of 33 schizophrenia and 29 control subjects underwent PET to measure the availability of GABAARs expressing the α5 subunit (concentrated on hippocampal somatostatin interneurons). We demonstrate-in the spatial memory task, during memory recall-that theta power increases in left medial temporal lobe (mTL) are impaired in schizophrenia, as is theta phase coupling between mPFC and mTL. Importantly, the latter cannot be explained by theta power changes, head movement, antipsychotics, cannabis use, or IQ, and is not found in other frequency bands. Moreover, mPFC-mTL theta coupling correlated strongly with performance in controls, but not in subjects with schizophrenia, who were mildly impaired at the spatial memory task and no better than chance on the memory integration task. Finally, mTL regions showing reduced phase coupling in schizophrenia magnetoencephalography participants overlapped substantially with areas of diminished α5-GABAAR availability in the wider schizophrenia PET sample. These results indicate that mPFC-mTL dysconnectivity in schizophrenia is due to a loss of theta phase coupling, and imply α5-GABAARs (and the cells that express them) have a role in this process.
Keywords: hippocampus; prefrontal cortex; schizophrenia; spatial memory; theta.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Anderson KL, Rajagovindan R, Ghacibeh GA, Meador KJ, Ding M.. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cereb Cortex 2010; 20: 1604–12. - PubMed
-
- Andreou C, Leicht G, Nolte G, Polomac N, Moritz S, Karow A, et al.Resting-state theta-band connectivity and verbal memory in schizophrenia and in the high-risk state. Schizophr Res 2015; 161: 299–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

