Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: a prospective, randomized, controlled first-in-human pilot study
- PMID: 32238172
- PMCID: PMC7110715
- DOI: 10.1186/s13018-020-01631-8
Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: a prospective, randomized, controlled first-in-human pilot study
Abstract
Background: This study tested the hypothesis that treatment of symptomatic, partial-thickness rotator cuff tears (sPTRCT) with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated from lipoaspirate at the point of care is safe and more effective than corticosteroid injection.
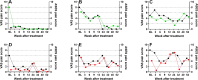
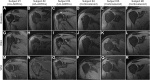
Methods: Subjects aged between 30 and 75 years with sPTRCT who did not respond to physical therapy treatments for at least 6 weeks were randomly assigned to receive a single injection of an average 11.4 × 106 UA-ADRCs (in 5 mL liquid; mean cell viability: 88%) (n = 11; modified intention-to-treat (mITT) population) or a single injection of 80 mg of methylprednisolone (40 mg/mL; 2 mL) plus 3 mL of 0.25% bupivacaine (n = 5; mITT population), respectively. Safety and efficacy were assessed using the American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form (ASES), RAND Short Form-36 Health Survey, and pain visual analogue scale (VAS) at baseline (BL) as well as 3 weeks (W3), W6, W9, W12, W24, W32, W40, and W52 post treatment. Fat-saturated T2-weighted magnetic resonance imaging of the shoulder was performed at BL as well as at W24 and W52 post treatment.
Results: No severe adverse events related to the injection of UA-ADRCs were observed in the 12 months post treatment. The risks connected with treatment of sPTRCT with UA-ADRCs were not greater than those connected with treatment of sPTRCT with corticosteroid injection. However, one subject in the corticosteroid group developed a full rotator cuff tear during the course of this pilot study. Despite the small number of subjects in this pilot study, those in the UA-ADRCs group showed statistically significantly higher mean ASES total scores at W24 and W52 post treatment than those in the corticosteroid group (p < 0.05).
Discussion: This pilot study suggests that the use of UA-ADRCs in subjects with sPTRCT is safe and leads to improved shoulder function without adverse effects. To verify the results of this initial safety and feasibility pilot study in a larger patient population, a randomized controlled trial on 246 patients suffering from sPTRCT is currently ongoing.
Trial registration: Clinicaltrials.gov ID NCT02918136. Registered September 28, 2016, https://clinicaltrials.gov/ct2/show/NCT02918136.
Level of evidence: Level I; prospective, randomized, controlled trial.
Keywords: Adipose-derived regenerative cells (ADRCs); Partial rotator cuff tear; Point of care treatment; Safety; Shoulder disease; Stem cells; Stromal vascular fraction.
Conflict of interest statement
JLH, TRF, JW, MH, MH, and ML are employees of Sanford Health, a minority shareholder of InGeneron, Inc. (Houston, TX, USA), which is the manufacturer and distributor of the Transpose RT/Matrase system that was investigated in this pilot study. GEW is employed as Chief Scientific Officer at InGeneron, Inc. CS has served as consultant to SciCoTec (Gruenwald, Germany), the majority shareholder of InGeneron, Inc. CA is managing director of InGeneron GmbH (Munich, Germany) which is owned by InGeneron, Inc. EA is Executive Chair of InGeneron, Inc., and Chairman of the Board of SciCoTec.
Figures


References
-
- Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990;254:64–74. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

