Angiomodulin (IGFBP7) is a cerebral specific angiocrine factor, but is probably not a blood-brain barrier inducer
- PMID: 32238174
- PMCID: PMC7110827
- DOI: 10.1186/s12987-020-00188-2
Angiomodulin (IGFBP7) is a cerebral specific angiocrine factor, but is probably not a blood-brain barrier inducer
Abstract
Background: Several secreted factors have been identified as drivers of cerebral vasculature development and inducers of blood-brain barrier (BBB) differentiation. Vascular endothelial growth factor A (VEGF-A) is central for driving cerebral angiogenesis and Wnt family factors (Wnt7a, Wnt7b and norrin) are central for induction and maintenance of barrier properties. Expressed by developing neural tissue (neuron and glia progenitors), they influence the formation of central nervous system (CNS) vascular networks. Another type of factors are tissue-specific paracrine factors produced by endothelial cells (ECs), also known as 'angiocrine' factors, that provide instructive signals to regulate homeostatic and regenerative processes. Very little is known about CNS angiocrine factors and their role in BBB development. Angiomodulin (AGM) was reported to be expressed by developing vasculature and by pathological tumor vasculature. Here we investigated AGM in the developing CNS and its function as a potential BBB inducer.
Methods: We analyzed microarray data to identify potential angiocrine factors specifically expressed at early stages of barrier formation. We then tested AGM expression with immunofluorescence and real-time PCR in various organs during development, post-natal and in adults. Permeability induction with recombinant proteins (Miles assay) was used to test potential interaction of AGM with VEGF-A.
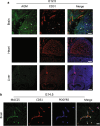
Results: Several angiocrine factors are differentially expressed by CNS ECs and AGM is a prominent CNS-specific angiocrine candidate. Contrary to previous reports, we found that AGM protein expression is specific to developing CNS endothelium and not to highly angiogenic developing vasculature in general. In skin vasculature we found that AGM antagonizes VEGF-A-induced vascular hyperpermeability. Finally, CNS AGM expression is not specific to BBB vasculature and AGM is highly expressed in non-BBB choroid-plexus vasculature.
Conclusions: We propose AGM as a developmental CNS vascular-specific marker. AGM is not a pan-endothelial marker, nor a general marker for developing angiogenic vasculature. Thus, AGM induction in the developing CNS might be distinct from its induction in pathology. While AGM is able to antagonize VEGF-A-induced vascular hyperpermeability in the skin, its high expression levels in non-BBB CNS vasculature does not support its potential role as a BBB inducer. Further investigation including loss-of-function approaches might elucidate AGM function in the developing CNS.
Keywords: Angiocrine factors; Angiomodulin (IGFBP7); Blood–brain barrier (BBB); Choroid plexus; Development; VEGF-A.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

