Lung function and microbiota diversity in cystic fibrosis
- PMID: 32238195
- PMCID: PMC7114784
- DOI: 10.1186/s40168-020-00810-3
Lung function and microbiota diversity in cystic fibrosis
Abstract
Background: Chronic infection and concomitant airway inflammation is the leading cause of morbidity and mortality for people living with cystic fibrosis (CF). Although chronic infection in CF is undeniably polymicrobial, involving a lung microbiota, infection surveillance and control approaches remain underpinned by classical aerobic culture-based microbiology. How to use microbiomics to direct clinical management of CF airway infections remains a crucial challenge. A pivotal step towards leveraging microbiome approaches in CF clinical care is to understand the ecology of the CF lung microbiome and identify ecological patterns of CF microbiota across a wide spectrum of lung disease. Assessing sputum samples from 299 patients attending 13 CF centres in Europe and the USA, we determined whether the emerging relationship of decreasing microbiota diversity with worsening lung function could be considered a generalised pattern of CF lung microbiota and explored its potential as an informative indicator of lung disease state in CF.
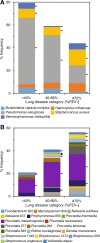
Results: We tested and found decreasing microbiota diversity with a reduction in lung function to be a significant ecological pattern. Moreover, the loss of diversity was accompanied by an increase in microbiota dominance. Subsequently, we stratified patients into lung disease categories of increasing disease severity to further investigate relationships between microbiota characteristics and lung function, and the factors contributing to microbiota variance. Core taxa group composition became highly conserved within the severe disease category, while the rarer satellite taxa underpinned the high variability observed in the microbiota diversity. Further, the lung microbiota of individual patient were increasingly dominated by recognised CF pathogens as lung function decreased. Conversely, other bacteria, especially obligate anaerobes, increasingly dominated in those with better lung function. Ordination analyses revealed lung function and antibiotics to be main explanators of compositional variance in the microbiota and the core and satellite taxa. Biogeography was found to influence acquisition of the rarer satellite taxa.
Conclusions: Our findings demonstrate that microbiota diversity and dominance, as well as the identity of the dominant bacterial species, in combination with measures of lung function, can be used as informative indicators of disease state in CF. Video Abstract.
Keywords: Antibiotics; Biogeography; Cystic fibrosis; Disease severity; Ecological patterns; Lung function; Lung microbiome; Lung microbiota; Microbial surveillance.
Conflict of interest statement
SMM is now an employee of Vertex Pharmaceuticals, and may hold stock and/or stock options in that company. JP has a paid consultancy with Next Gen Diagnostics LLC. The other authors have no conflicts to declare.
Figures



References
-
- Anon . Cystic Fibrosis Foundation Patient Registry 2017 Annual Data Report. Bethesda, Maryland: Cystic Fibrosis Foundation; 2018.
-
- Anon . UK Cystic Fibrosis Registry Annual Data Report 2017. London: Cystic Fibrosis Trust; 2018.
-
- Bush A, Bilton D, Hodson M. Hodson and Geddes’ Cystic Fibrosis. 4. Boca Raton: CRC Press; 2016.
-
- Berger M. Inflammation in the lung in cystic fibrosis. A vicious cycle that does more harm than good? Clin Rev Allergy. 1991;9:119–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

