Cinnamaldehyde protects against rat intestinal ischemia/reperfusion injuries by synergistic inhibition of NF-κB and p53
- PMID: 32238887
- PMCID: PMC7609352
- DOI: 10.1038/s41401-020-0359-9
Cinnamaldehyde protects against rat intestinal ischemia/reperfusion injuries by synergistic inhibition of NF-κB and p53
Abstract
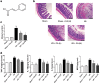
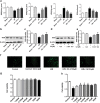
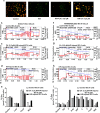
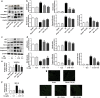
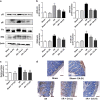
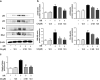
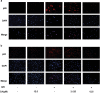
Our preliminary study shows that cinnamaldehyde (CA) could protect against intestinal ischemia/reperfusion (I/R) injuries, in which p53 and NF-κB p65 play a synergistic role. In this study, we conducted in vivo and in vitro experiments to verify this proposal. SD rats were pretreated with CA (10 or 40 mg · kg-1 · d-1, ig) for 3 days, then subjected to 1 h mesenteric ischemia followed by 2 h reperfusion. CA pretreatment dose-dependently ameliorated morphological damage and reduced inflammation evidenced by decreased TNF-α, IL-1β, and IL-6 levels and MPO activity in I/R-treated intestinal tissues. CA pretreatment also attenuated oxidative stress through restoring SOD, GSH, LDH, and MDA levels in I/R-treated intestinal tissues. Furthermore, CA pretreatment significantly reduced the expression of inflammation/apoptosis-related NF-κB p65, IKKβ, IK-α, and NF-κB p50, and downregulated apoptotic protein expression including p53, Bax, caspase-9 and caspase-3, and restoring Bcl-2, in I/R-treated intestinal tissues. We pretreated IEC-6 cells in vitro with CA for 24 h, followed by 4 h hypoxia and 3 h reoxygenation (H/R) incubation. Pretreatment with CA (3.125, 6.25, and 12.5 μmol · L-1) significantly reversed H/R-induced reduction of IEC-6 cell viability. CA pretreatment significantly suppressed oxidative stress, NF-κB activation and apoptosis in H/R-treated IEC-6 cells. Moreover, CA pretreatment significantly reversed mitochondrial dysfunction in H/R-treated IEC-6 cells. CA pretreatment inhibited the nuclear translocation of p53 and NF-κB p65 in H/R-treated IEC-6 cells. Double knockdown or overexpression of p53 and NF-κB p65 caused a synergistic reduction or elevation of p53 compared with knockdown or overexpression of p53 or NF-κB p65 alone. In H/R-treated IEC-6 cells with double knockdown or overexpression of NF-κB p65 and p53, CA pretreatment caused neither further decrease nor increase of NF-κB p65 or p53 expression, suggesting that CA-induced synergistic inhibition on both NF-κB and p53 played a key role in ameliorating intestinal I/R injuries. Finally, we used immunoprecipitation assay to demonstrate an interaction between p53 and NF-κB p65, showing the basis for CA-induced synergistic inhibition. Our results provide valuable information for further studies.
Keywords: NF-κB; apoptosis; cinnamaldehyde; inflammation; mesenteric ischemia/reperfusion injury; mitochondria; oxidative stress; p53.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Gao S, Zhu Y, Li H, Xia Z, Wu Q, Yao S, et al. Remote ischemic postconditioning protects against renal ischemia/reperfusion injury by activation of T-LAK-cell-originated protein kinase (TOPK)/PTEN/Akt signaling pathway mediated anti-oxidation and anti-inflammation. Int Immunopharmacol. 2016;38:395–401. - PubMed
-
- Gonul Y, Ozsoy M, Kocak A, Ozkececi ZT, Karavelioglu A, Bozkurt MF, et al. Antioxidant, antiapoptotic and inflammatory effects of interleukin-18 binding protein on kidney damage induced by hepatic ischemia reperfusion. Am J Med Sci. 2016;351:607–15. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

