Pathology, diagnostics, and classification of medulloblastoma
- PMID: 32239782
- PMCID: PMC7317787
- DOI: 10.1111/bpa.12837
Pathology, diagnostics, and classification of medulloblastoma
Abstract
Medulloblastoma (MB) is the most common CNS embryonal tumor. While the overall cure rate is around 70%, patients with high-risk disease continue to have poor outcome and experience long-term morbidity. MB is among the tumors for which diagnosis, risk stratification, and clinical management has shown the most rapid advancement. These advances are largely due to technological improvements in diagnosis and risk stratification which now integrate histomorphologic classification and molecular classification. MB stands as a prototype for other solid tumors in how to effectively integrate morphology and genomic data to stratify clinicopathologic risk and aid design of innovative clinical trials for precision medicine. This review explores the current diagnostic and classification of MB in modern neuropathology laboratories.
Keywords: Group 3; Group 4; SHH; WNT; classification; diagnosis; histology; medulloblastoma; neuropathology; non-WNT/non-SHH.
© 2020 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The author has not conflict of interest to declare.
Figures
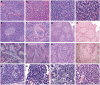

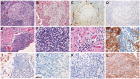


References
-
- Andrey K, Lukas C, Northcott PA, Tanvi S, Marina R, Jones DTW et al (2017) DNA‐methylation profiling discloses significant advantages over NanoString method for molecular classification of medulloblastoma. Acta Neuropathol 134:965–967. - PubMed
-
- Arceci RJ (2010) Adult and pediatric medulloblastomas are genetically distinct and require different algorithms for molecular risk stratification. Yearb Oncol 28:203–204. - PubMed
-
- Bailey P (1925) Medulloblastoma cerebelli. Arch Neurol Psychiatry 14:192–224.
-
- Bailey P, Cushing H (1926) A Classification of the Tumors of the Glioma Group on a Histogenetic Basis With a Correlated Study of Prognosis. Lippincott: Philadelphia.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

