HIV testing and treatment coverage achieved after 4 years across 14 urban and peri-urban communities in Zambia and South Africa: An analysis of findings from the HPTN 071 (PopART) trial
- PMID: 32240156
- PMCID: PMC7117659
- DOI: 10.1371/journal.pmed.1003067
HIV testing and treatment coverage achieved after 4 years across 14 urban and peri-urban communities in Zambia and South Africa: An analysis of findings from the HPTN 071 (PopART) trial
Abstract
Background: In 2014, the Joint United Nations Programme on HIV/AIDS (UNAIDS) set the 90-90-90 targets: that 90% of people living with HIV know their HIV status, that 90% of those who know their HIV-positive status are on antiretroviral therapy (ART), and that 90% of those on treatment are virally suppressed. The aim was to reach these targets by 2020. We assessed the feasibility of achieving the first two targets, and the corresponding 81% ART coverage target, as part of the HIV Prevention Trials Network (HPTN) 071 Population Effects of Antiretroviral Therapy to Reduce HIV Transmission (PopART) community-randomized trial.
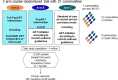
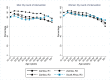
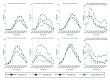
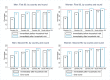
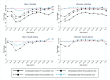
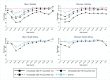
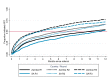
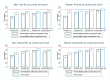
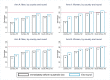
Methods and findings: The study population was individuals aged ≥15 years living in 14 urban and peri-urban "PopART intervention" communities in Zambia and South Africa (SA), with a total population of approximately 600,000 and approximately 15% adult HIV prevalence. Community HIV care providers (CHiPs) delivered the PopART intervention during 2014-2017. This was a combination HIV prevention package including universal home-based HIV testing, referral of HIV-positive individuals to government HIV clinic services that offered universal ART (Arm A) or ART according to national guidelines (Arm B), and revisits to HIV-positive individuals to support linkage to HIV care and retention on ART. The intervention was delivered in 3 "rounds," each about 15 months long, during which CHiPs visited all households and aimed to contact all individuals aged ≥15 years at least once. In Arm A in Round 3 (R3), 67% (41,332/61,402) of men and 86% (56,345/65,896) of women in Zambia and 56% (17,813/32,095) of men and 71% (24,461/34,514) of women in SA participated in the intervention, among 193,907 residents aged ≥15 years. Following participation, HIV status was known by 90% of men and women in Zambia and by 78% of men and 85% of women in SA. The median time from CHiP referral of HIV-positive individuals to ART initiation was approximately 3 months. By the end of R3, an estimated 95% of HIV-positive women and 85% of HIV-positive men knew their HIV status, and among these individuals, approximately 90% of women and approximately 85% of men were on ART. ART coverage among all HIV-positive individuals was approximately 85% in women and approximately 75% in men, up from about 45% at the start of the study. ART coverage was lowest among men aged 18 to 34 and women aged 15 to 24 years, and among mobile individuals/in-migrants. Findings from Arm B were similar. The main limitations to our study were that estimates of testing and treatment coverage among men relied on considerable extrapolation because, in each round, approximately one-third of men did not participate in the PopART intervention; that our findings are for a service delivery model that was relatively intensive; and that we did not have comparable data from the 7 "standard-of-care" (Arm C) communities.
Conclusions: Our study showed that very high HIV testing and treatment coverage can be achieved through persistent delivery of universal testing, facilitated linkage to HIV care, and universal treatment services. The ART coverage target of 81% was achieved overall, after 4 years of delivery of the PopART intervention, though important gaps remained among men and young people. Our findings are consistent with previously reported findings from southern and east Africa, extending their generalisability to urban settings with high rates of in-migration and mobility and to Zambia and SA.
Trial registration: ClinicalTrials.gov NCT01900977.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: HA is a member of the technical review panel for the Global Fund.
Figures

Similar articles
-
Achieving the UNAIDS 90-90-90 targets: a comparative analysis of four large community randomised trials delivering universal testing and treatment to reduce HIV transmission in sub-Saharan Africa.BMC Public Health. 2022 Dec 13;22(1):2333. doi: 10.1186/s12889-022-14713-5. BMC Public Health. 2022. PMID: 36514036 Free PMC article.
-
Towards 90-90: Findings after two years of the HPTN 071 (PopART) cluster-randomized trial of a universal testing-and-treatment intervention in Zambia.PLoS One. 2018 Aug 10;13(8):e0197904. doi: 10.1371/journal.pone.0197904. eCollection 2018. PLoS One. 2018. PMID: 30096139 Free PMC article. Clinical Trial.
-
A universal testing and treatment intervention to improve HIV control: One-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial.PLoS Med. 2017 May 2;14(5):e1002292. doi: 10.1371/journal.pmed.1002292. eCollection 2017 May. PLoS Med. 2017. PMID: 28464041 Free PMC article. Clinical Trial.
-
Tuberculosis prevalence after 4 years of population-wide systematic TB symptom screening and universal testing and treatment for HIV in the HPTN 071 (PopART) community-randomised trial in Zambia and South Africa: A cross-sectional survey (TREATS).PLoS Med. 2023 Sep 8;20(9):e1004278. doi: 10.1371/journal.pmed.1004278. eCollection 2023 Sep. PLoS Med. 2023. PMID: 37682971 Free PMC article. Clinical Trial.
-
Implementation of Universal HIV Testing and Treatment to Reduce HIV Incidence in Botswana: the Ya Tsie Study.Curr HIV/AIDS Rep. 2020 Oct;17(5):478-486. doi: 10.1007/s11904-020-00523-0. Curr HIV/AIDS Rep. 2020. PMID: 32797382 Review.
Cited by
-
Does enhanced HIV prevention, diagnosis, and linkage to care reduce hospitalisation in high HIV-burden communities in Zambia and South Africa? findings from the HPTN 071 (PopART) randomised trial.PLOS Glob Public Health. 2025 May 8;5(5):e0004373. doi: 10.1371/journal.pgph.0004373. eCollection 2025. PLOS Glob Public Health. 2025. PMID: 40339051 Free PMC article.
-
The impact of a combined TB/HIV intervention on the incidence of TB infection among adolescents and young adults in the HPTN 071 (PopART) trial communities in Zambia and South Africa.PLOS Glob Public Health. 2023 Jul 14;3(7):e0001473. doi: 10.1371/journal.pgph.0001473. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 37450474 Free PMC article.
-
The impact of an innovative community-based peer-led intervention on uptake and coverage of sexual and reproductive health services among adolescents and young people 15-24 years old: results from the Yathu Yathu cluster randomised trial.BMC Public Health. 2024 May 28;24(1):1424. doi: 10.1186/s12889-024-18894-z. BMC Public Health. 2024. PMID: 38807091 Free PMC article. Clinical Trial.
-
Achieving the UNAIDS 90-90-90 targets: a comparative analysis of four large community randomised trials delivering universal testing and treatment to reduce HIV transmission in sub-Saharan Africa.BMC Public Health. 2022 Dec 13;22(1):2333. doi: 10.1186/s12889-022-14713-5. BMC Public Health. 2022. PMID: 36514036 Free PMC article.
-
Characterizing the HIV care continuum among children and adolescents with HIV in eastern and southern Africa in the era of "Universal Test and Treat": A systematic review and meta-analysis.J Int AIDS Soc. 2025 Jun;28(6):e26526. doi: 10.1002/jia2.26526. J Int AIDS Soc. 2025. PMID: 40515449 Free PMC article. Review.
References
-
- UNAIDS. An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: 2014.
-
- Radin E. What does population data tell us about programming priorities? Insights from 14 countries. IAS; Amsterdam, July 22–27, 2018.
-
- UNAIDS. Global update; Communities at the Centre. Geneva, Switzerland: 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

