Project YES! Youth Engaging for Success: A randomized controlled trial assessing the impact of a clinic-based peer mentoring program on viral suppression, adherence and internalized stigma among HIV-positive youth (15-24 years) in Ndola, Zambia
- PMID: 32240186
- PMCID: PMC7117673
- DOI: 10.1371/journal.pone.0230703
Project YES! Youth Engaging for Success: A randomized controlled trial assessing the impact of a clinic-based peer mentoring program on viral suppression, adherence and internalized stigma among HIV-positive youth (15-24 years) in Ndola, Zambia
Erratum in
-
Correction: Project YES! Youth Engaging for Success: A randomized controlled trial assessing the impact of a clinic-based peer mentoring program on viral suppression, adherence and internalized stigma among HIV-positive youth (15-24 years) in Ndola, Zambia.PLoS One. 2020 Apr 23;15(4):e0232488. doi: 10.1371/journal.pone.0232488. eCollection 2020. PLoS One. 2020. PMID: 32324830 Free PMC article.
Abstract
Background: Youth-led strategies remain untested in clinic-based programs to improve viral suppression (VS) and reduce stigma among HIV-positive adolescents and young adults (AYA) in sub-Saharan Africa. In response, Project YES! placed paid HIV-positive youth peer mentors (YPM) in four HIV clinics in Ndola, Zambia including a Children's Hospital (pediatric setting), an adult Hospital and two primary care facilities (adult settings).
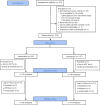
Methods: A randomized controlled trial was conducted from December 2017 to February 2019. Consecutively recruited 15 to 24-year-olds were randomly assigned to an intervention arm with monthly YPM one-on-one and group sessions and optional caregiver support groups, or a usual care comparison arm. Survey data and blood samples were collected at baseline and at the six-month midline. Generalized estimating equation models evaluated the effect of study arm over time on VS, antiretroviral treatment (ART) adherence gap, and internalized stigma.
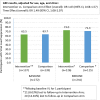
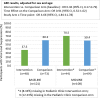
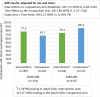
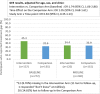
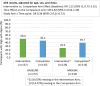
Results: Out of 276 randomized youth, 273 were included in the analysis (Intervention n = 137, Comparison n = 136). VS significantly improved in both arms (I:63.5% to 73.0%; C:63.7% to 71.3.0%) [OR:1.49, 95% CI:1.08, 2.07]. In a stratified analysis intervention (I:37.5% to 70.5%) versus the comparison (C:60.3% to 59.4%) participants from the pediatric clinic experienced a relative increase in the odds of VS by a factor of 4.7 [interaction term OR:4.66, 95% CI:1.84, 11.78]. There was no evidence of a study arm difference in VS among AYA in adult clinics, or in ART adherence gaps across clinics. Internalized stigma significantly reduced by a factor of 0.39 [interaction term OR:0.39, 95% CI:0.21,0.73] in the intervention (50.4% to 25.4%) relative to the comparison arm (45.2% to 39.7%).
Conclusions: Project YES! engaged AYA, improving VS in the pediatric clinic and internalized stigma in the pediatric and adult clinics. Further research is needed to understand the intersection of VS and internalized stigma among AYA attending adult HIV clinics.
Trial registration: ClinicalTrials.gov NCT04115813.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
"Adolescents do not only require ARVs and adherence counseling": A qualitative investigation of health care provider experiences with an HIV youth peer mentoring program in Ndola, Zambia.PLoS One. 2021 Jun 9;16(6):e0252349. doi: 10.1371/journal.pone.0252349. eCollection 2021. PLoS One. 2021. PMID: 34106967 Free PMC article.
-
"Project YES! has given me a task to reach undetectable": Qualitative findings from a peer mentoring program for youth living with HIV in Zambia.PLoS One. 2023 Oct 13;18(10):e0292719. doi: 10.1371/journal.pone.0292719. eCollection 2023. PLoS One. 2023. PMID: 37831675 Free PMC article. Clinical Trial.
-
Experiences with a violence and mental health safety protocol for a randomized controlled trial to support youth living with HIV.Glob Health Res Policy. 2021 Oct 15;6(1):40. doi: 10.1186/s41256-021-00224-0. Glob Health Res Policy. 2021. PMID: 34654487 Free PMC article.
-
Interventions to Improve Antiretroviral Therapy Adherence Among Adolescents and Youth in Low- and Middle-Income Countries: A Systematic Review 2015-2019.AIDS Behav. 2020 Oct;24(10):2797-2810. doi: 10.1007/s10461-020-02822-4. AIDS Behav. 2020. PMID: 32152815 Free PMC article.
-
Alcohol Use and Antiretroviral Therapy Non-Adherence Among Adults Living with HIV/AIDS in Sub-Saharan Africa: A Systematic Review and Meta-Analysis.AIDS Behav. 2020 Jun;24(6):1727-1742. doi: 10.1007/s10461-019-02716-0. AIDS Behav. 2020. PMID: 31673913 Free PMC article.
Cited by
-
Integrating Adolescent Mental Health into HIV Prevention and Treatment Programs: Can Implementation Science Pave the Path Forward?AIDS Behav. 2023 May;27(Suppl 1):145-161. doi: 10.1007/s10461-022-03876-2. Epub 2022 Nov 2. AIDS Behav. 2023. PMID: 36322219 Free PMC article.
-
Adolescent support club attendance and self-efficacy associated with HIV treatment outcomes in Tanzania.PLOS Glob Public Health. 2022 Oct 3;2(10):e0000065. doi: 10.1371/journal.pgph.0000065. eCollection 2022. PLOS Glob Public Health. 2022. PMID: 36962483 Free PMC article.
-
A multilevel health system intervention for virological suppression in adolescents and young adults living with HIV in rural Kenya and Uganda (SEARCH-Youth): a cluster randomised trial.Lancet HIV. 2023 Aug;10(8):e518-e527. doi: 10.1016/S2352-3018(23)00118-2. Lancet HIV. 2023. PMID: 37541706 Free PMC article. Clinical Trial.
-
Interventions to Improve Adolescent HIV Care Outcomes.Curr HIV/AIDS Rep. 2023 Aug;20(4):218-230. doi: 10.1007/s11904-023-00663-z. Epub 2023 Jun 10. Curr HIV/AIDS Rep. 2023. PMID: 37300592 Free PMC article. Review.
-
Advocacy for patients with headache disorders.eNeurologicalSci. 2023 May 10;31:100466. doi: 10.1016/j.ensci.2023.100466. eCollection 2023 Jun. eNeurologicalSci. 2023. PMID: 37250108 Free PMC article. Review.
References
-
- Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. Journal of acquired immune deficiency syndromes (1999). 2009;51(1):65–71. Epub 2009/03/14. 10.1097/QAI.0b013e318199072e - DOI - PMC - PubMed
-
- Ding H, Wilson CM, Modjarrad K, McGwin G, Tang J, Vermund SH. Predictors of Suboptimal Virologic Response to Highly Active Antiretroviral Therapy Among Human Immunodeficiency Virus–Infected Adolescents: Analyses of the Reaching for Excellence in Adolescent Care and Health (REACH) Project. Archives of pediatrics & adolescent medicine. 2009;163(12):1100–5. - PMC - PubMed
-
- Jobanputra K, Parker LA, Azih C, Okello V, Maphalala G, Kershberger B, et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS One. 2015;10(2):e0116144 10.1371/journal.pone.0116144 - DOI - PMC - PubMed
-
- World Health Organzation. HIV and adolescents: guidance for HIV testing and counselling and care for adolescents living with HIV 2013 [cited 2016 February 11, 2016]. Available from: http://apps.who.int/iris/bitstream/10665/94334/1/9789241506168_eng.pdf?ua=1.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

