Spanish real-world experience with fingolimod in relapsing-remitting multiple sclerosis patients: MS NEXT study
- PMID: 32240213
- PMCID: PMC7117743
- DOI: 10.1371/journal.pone.0230846
Spanish real-world experience with fingolimod in relapsing-remitting multiple sclerosis patients: MS NEXT study
Abstract
Purpose: The objective of this study was to characterize the demographic and clinical profile of RRMS patients receiving fingolimod in Spain, and to evaluate drug effectiveness and safety in clinical practice.
Methods: This observational, retrospective, multicentre, nationwide study was performed at 56 Spanish hospitals and involved 804 RRMS patients who received oral fingolimod (0.5 mg) since November 2011, with a minimum follow-up of 12 months.
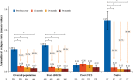
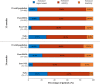
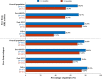
Results: The mean annualized relapse rate (ARR) in the year before fingolimod was 1.08 and the median EDSS was 3; patients were exposed to fingolimod for 2.2 years as average; regarding magnetic resonance imaging (MRI) activity, more than half of the patients had >20 lesions at baseline. Patients were previously treated with first-line injectable DMTs (60.3%), or natalizumab (31.3%), and 8.3% were naïve patients. Overall, the ARR significantly decreased to 0.28, 0.22 and 0.17 (74.1%, 79.7% and 83.5% of relative reduction, respectively) after 12, 24 and 36 months of treatment, P<0.001. The ARR of patients who switched from natalizumab to fingolimod was stable over the study. Most of the patients (88.7%) were free from confirmed disability and MRI activity (67.3%) after 24 months. The persistence after 12 months on fingolimod was 93.9%.
Conclusions: The subgroups of patients analysed showed differential baseline demographic and clinical characteristics. The analysis of patients who received fingolimod in routine clinical practice confirmed adequate efficacy and safety, even for long-term treatment. The present data also confirmed the positive benefit/risk balance with fingolimod in real-world clinical practice setting.
Conflict of interest statement
Authors have read the journal's policy and the authors of this manuscript have the following competing interests: - Francisco Barrero has received consulting or speaking fees from Almirall, Biogen, Genzyme, Merck Serono, Novartis, Roche, Sanofi and Teva. Francisco Barrero discloses no conflict of interest in the elaboration of this paper. - Javier Mallada-Frechin has received consulting or speaking fees from Almirall, Biogen, Genzyme, Merck Serono, Novartis, Roche, Sanofi and Teva. Javier Mallada-Frechin discloses no conflict of interest in the elaboration of this paper. - Maria Luisa Martínez-Ginés discloses no conflict of interest. - María Eugenia Marzo discloses no conflict of interest. - Virginia Meca-Lallana has received consulting or speaking fees from Almirall, Biogen, Genzyme, Merck Serono, Novartis, Roche, Terumo, Sanofi and Teva. Virginia Meca-Lallana discloses no conflict of interest in the elaboration of this paper. Response to Reviewers - Guillermo Izquierdo has received consulting or speaking fees from Actelion, Almirall, Bayer, Biogen-Idec, Celgene, Merck Serono, Novartis, Roche, Sanofi and Teva; and research support from Almirall, Bayer, Biogen Idec, Merck Serono, Novartis, Sanofi and Teva. Guillermo Izquierdo discloses no conflict of interest in the elaboration of this paper. - José Ramón Ara has received consulting honoraria from Genzyme, Novartis and Sanofi; and honoraria for lecturing or travel expenses for attending meetings from Biogen Idec, Genzyme, Novartis and Sanofi-Aventis. José Ramón Ara discloses no conflict of interest in the elaboration of this paper. - Celia Oreja-Guevara has received consulting or speaking fees from Actelion, Biogen, Merck Serono, Genzyme, Novartis, Roche, Sanofi, Teva, and Novartis. Celia Oreja-Guevara discloses no conflict of interest in the elaboration of this paper. - José Meca-Lallana has received consulting or speaking fees from Biogen, Celgene, Genzyme, Merck Serono, Novartis, Roche, and Teva. José Meca-Lallana discloses no conflict of interest in the elaboration of this paper. - Lucía Forero has received consulting or speaking fees from Biogen, Merck Serono, Novartis, Roche and Sanofi. Lucía Forero discloses no conflict of interest in the elaboration of this paper. - ISV and MJM are employed by Novartis Farmacéutica, S.A. There are no patents, products in development or marketed products to declare. The ISV and MJM’s commercial affiliation do not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Chaudhuri A. Multiple sclerosis is primarily a neurodegenerative disease. J Neural Transm (Vienna). 2013; 120: 1463–6. - PubMed

