1H, 13C, and 15N Backbone assignments of the human brain and acute leukemia cytoplasmic (BAALC) protein
- PMID: 32240523
- PMCID: PMC7462906
- DOI: 10.1007/s12104-020-09938-7
1H, 13C, and 15N Backbone assignments of the human brain and acute leukemia cytoplasmic (BAALC) protein
Abstract
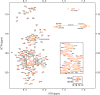
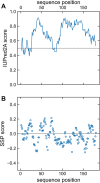
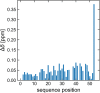
The brain and acute leukemia cytoplasmic (BAALC; UniProt entry Q8WXS3) is a 180-residue-long human protein having six known isoforms. BAALC is expressed in either hematopoietic or neuroectodermal cells and its specific function is still to be revealed. However, as a presumably membrane-anchored protein at the cytoplasmic side it is speculated that BAALC exerts its function at the postsynaptic densities of certain neurons and might play a role in developing cytogenetically normal acute myeloid leukemia (CN-AML) when it is highly overexpressed by myeloid or lymphoid progenitor cells. In order to better understand the physiological role of BAALC and to provide the basis for a further molecular characterization of BAALC, we report here the 1H, 13C, and 15N resonance assignments for the backbone nuclei of its longest hematopoietic isoform (isoform 1). In addition, we present a 1HN and 15NH chemical shift comparison of BAALC with its shortest, neuroectodermal isoform (isoform 6) which shows only minor changes in the 1H and 15N chemical shifts.
Keywords: Brain and acute leukemia cytoplasmic protein (BAALC); Heteronuclear NMR; Intrinsically disordered protein (IDP); Neuroectodermal and hematopoietic cell function; Resonance assignments.
Figures

References
-
- Baldus CD, Tanner SM, Ruppert AS, Whitman SP, Archer KJ, Marcucci G, Caligiuri MA, Carroll AJ, Vardiman JW, Powell BL, Allen SL, Moore JO, Larson RA, Kolitz JE, de la Chapelle A, Bloomfield CD. BAALC expression predicts clinical outcome of de novo acute myeloid leukemia patients with normal cytogenetics: a Cancer and Leukemia Group B Study. Blood. 2003;102:1613–1618. doi: 10.1182/blood-2003-02-0359. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

