Imaging Guidance for Therapeutic Delivery: The Dawn of Neuroenergetics
- PMID: 32240530
- PMCID: PMC7283376
- DOI: 10.1007/s13311-020-00843-4
Imaging Guidance for Therapeutic Delivery: The Dawn of Neuroenergetics
Erratum in
-
Correction to: Imaging Guidance for Therapeutic Delivery: the Dawn of Neuroenergetics.Neurotherapeutics. 2020 Oct;17(4):2090. doi: 10.1007/s13311-020-00863-0. Neurotherapeutics. 2020. PMID: 32367474 Free PMC article. No abstract available.
Abstract
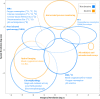
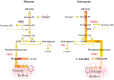
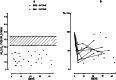
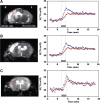
Modern neurocritical care relies on ancillary diagnostic testing in the form of multimodal monitoring to address acute changes in the neurological homeostasis. Much of our armamentarium rests upon physiological and biochemical surrogates of organ or regional level metabolic activity, of which a great deal is invested at the metabolic-hemodynamic-hydrodynamic interface to rectify the traditional intermediaries of glucose consumption. Despite best efforts to detect cellular neuroenergetics, current modalities cannot appreciate the intricate coupling between astrocytes and neurons. Invasive monitoring is not without surgical complication, and noninvasive strategies do not provide an adequate spatial or temporal resolution. Without knowledge of the brain's versatile behavior in specific metabolic states (glycolytic vs oxidative), clinical practice would lag behind laboratory empiricism. Noninvasive metabolic imaging represents a new hope in delineating cellular, nigh molecular level energy exchange to guide targeted management in a diverse array of neuropathology.
Keywords: Brain metabolism; MRI; Metabolic imaging; Neurocritical care; Neuroenergetics; Stroke.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

