Nanoparticle Delivery of Proangiogenic Transcription Factors into the Neonatal Circulation Inhibits Alveolar Simplification Caused by Hyperoxia
- PMID: 32240596
- PMCID: PMC7328311
- DOI: 10.1164/rccm.201906-1232OC
Nanoparticle Delivery of Proangiogenic Transcription Factors into the Neonatal Circulation Inhibits Alveolar Simplification Caused by Hyperoxia
Abstract
Rationale: Advances in neonatal critical care have greatly improved the survival of preterm infants, but the long-term complications of prematurity, including bronchopulmonary dysplasia (BPD), cause mortality and morbidity later in life. Although VEGF (vascular endothelial growth factor) improves lung structure and function in rodent BPD models, severe side effects of VEGF therapy prevent its use in patients with BPD.Objectives: To test whether nanoparticle delivery of proangiogenic transcription factor FOXM1 (forkhead box M1) or FOXF1 (forkhead box F1), both downstream targets of VEGF, can improve lung structure and function after neonatal hyperoxic injury.Methods: Newborn mice were exposed to 75% O2 for the first 7 days of life before being returned to a room air environment. On Postnatal Day 2, polyethylenimine-(5) myristic acid/polyethylene glycol-oleic acid/cholesterol nanoparticles containing nonintegrating expression plasmids with Foxm1 or Foxf1 cDNAs were injected intravenously. The effects of the nanoparticles on lung structure and function were evaluated using confocal microscopy, flow cytometry, and the flexiVent small-animal ventilator.Measurements and Main Results: The nanoparticles efficiently targeted endothelial cells and myofibroblasts in the alveolar region. Nanoparticle delivery of either FOXM1 or FOXF1 did not protect endothelial cells from apoptosis caused by hyperoxia but increased endothelial proliferation and lung angiogenesis after the injury. FOXM1 and FOXF1 improved elastin fiber organization, decreased alveolar simplification, and preserved lung function in mice reaching adulthood.Conclusions: Nanoparticle delivery of FOXM1 or FOXF1 stimulates lung angiogenesis and alveolarization during recovery from neonatal hyperoxic injury. Delivery of proangiogenic transcription factors has promise as a therapy for BPD in preterm infants.
Keywords: FOX transcription factors; VEGF signaling; bronchopulmonary dysplasia; nanoparticle gene delivery systems; neonatal hyperoxic lung injury.
Figures
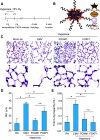
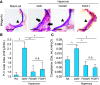
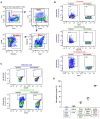

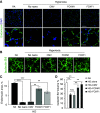

Comment in
-
Nanoparticle Delivery of Angiogenic Gene Therapy. Save the Vessels, Save the Lung!Am J Respir Crit Care Med. 2020 Jul 1;202(1):13-14. doi: 10.1164/rccm.202004-0933ED. Am J Respir Crit Care Med. 2020. PMID: 32338996 Free PMC article. No abstract available.
Similar articles
-
Suppression of inflammatory cell trafficking and alveolar simplification by the heme oxygenase-1 product carbon monoxide.Am J Physiol Lung Cell Mol Physiol. 2014 Apr 15;306(8):L749-63. doi: 10.1152/ajplung.00236.2013. Epub 2014 Feb 14. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24532288 Free PMC article.
-
Foxm1 regulates resolution of hyperoxic lung injury in newborns.Am J Respir Cell Mol Biol. 2015 May;52(5):611-21. doi: 10.1165/rcmb.2014-0091OC. Am J Respir Cell Mol Biol. 2015. PMID: 25275225 Free PMC article.
-
Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats.Am J Physiol Lung Cell Mol Physiol. 2005 Oct;289(4):L529-35. doi: 10.1152/ajplung.00336.2004. Epub 2005 May 20. Am J Physiol Lung Cell Mol Physiol. 2005. PMID: 15908474
-
Inhibition of β-catenin signaling improves alveolarization and reduces pulmonary hypertension in experimental bronchopulmonary dysplasia.Am J Respir Cell Mol Biol. 2014 Jul;51(1):104-13. doi: 10.1165/rcmb.2013-0346OC. Am J Respir Cell Mol Biol. 2014. PMID: 24484510
-
The lung alveolar lipofibroblast: an evolutionary strategy against neonatal hyperoxic lung injury.Antioxid Redox Signal. 2014 Nov 1;21(13):1893-904. doi: 10.1089/ars.2013.5793. Epub 2014 Mar 12. Antioxid Redox Signal. 2014. PMID: 24386954 Free PMC article. Review.
Cited by
-
Novel FOXF1-Stabilizing Compound TanFe Stimulates Lung Angiogenesis in Alveolar Capillary Dysplasia.Am J Respir Crit Care Med. 2023 Apr 15;207(8):1042-1054. doi: 10.1164/rccm.202207-1332OC. Am J Respir Crit Care Med. 2023. PMID: 36480964 Free PMC article.
-
FOXF1 Regulates Alveolar Epithelial Morphogenesis through Transcriptional Activation of Mesenchymal WNT5A.Am J Respir Cell Mol Biol. 2023 Apr;68(4):430-443. doi: 10.1165/rcmb.2022-0191OC. Am J Respir Cell Mol Biol. 2023. PMID: 36542853 Free PMC article.
-
Non-coding RNAs as Regulators of Cellular Senescence in Idiopathic Pulmonary Fibrosis and Chronic Obstructive Pulmonary Disease.Front Med (Lausanne). 2020 Dec 23;7:603047. doi: 10.3389/fmed.2020.603047. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33425948 Free PMC article. Review.
-
Protocol for minicircle production for gene therapy without subsequent cleanup steps.STAR Protoc. 2025 Jul 24;6(3):103982. doi: 10.1016/j.xpro.2025.103982. Online ahead of print. STAR Protoc. 2025. PMID: 40711875 Free PMC article.
-
Endothelial progenitor cells stimulate neonatal lung angiogenesis through FOXF1-mediated activation of BMP9/ACVRL1 signaling.Nat Commun. 2022 Apr 19;13(1):2080. doi: 10.1038/s41467-022-29746-y. Nat Commun. 2022. PMID: 35440116 Free PMC article.
References
-
- Ohtani S, Iwamaru A, Deng W, Ueda K, Wu G, Jayachandran G, et al. Tumor suppressor 101F6 and ascorbate synergistically and selectively inhibit non–small cell lung cancer growth by caspase-independent apoptosis and autophagy. Cancer Res. 2007;67:6293–6303. - PubMed
-
- Horiguchi M, Kojima H, Sakai H, Kubo H, Yamashita C. Pulmonary administration of integrin-nanoparticles regenerates collapsed alveoli. J Control Release. 2014;187:167–174. - PubMed
-
- Naderi N, Karponis D, Mosahebi A, Seifalian AM. Nanoparticles in wound healing; from hope to promise, from promise to routine. Front Biosci. 2018;23:1038–1059. - PubMed
-
- Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol. 2010;661:323–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

