Metabolic transcriptional memory
- PMID: 32240621
- PMCID: PMC7300383
- DOI: 10.1016/j.molmet.2020.01.019
Metabolic transcriptional memory
Abstract
Background: Organisms can be primed by metabolic exposures to continue expressing response genes even once the metabolite is no longer available, and can affect the speed and magnitude of responsive gene expression during subsequent exposures. This "metabolic transcriptional memory" can have a profound impact on the survivability of organisms in fluctuating environments.
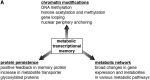
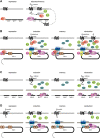
Scope of review: Here I present several examples of metabolic transcriptional memory in the microbial world and discuss what is known so far regarding the underlying mechanisms, which mainly focus on chromatin modifications, protein inheritance, and broad changes in metabolic network. From these lessons learned in microbes, some insights into the yet understudied human metabolic memory can be gained. I thus discuss the implications of metabolic memory in disease progression in humans - i.e., the memory of high blood sugar exposure and the resulting effects on diabetic complications.
Major conclusions: Carbon source shifts from glucose to other less preferred sugars such as lactose, galactose, and maltose for energy metabolism as well as starvation of a signal transduction precursor sugar inositol are well-studied examples of metabolic transcriptional memory in Escherichia coli and Saccharomyces cerevisiae. Although the specific factors guiding metabolic transcriptional memory are not necessarily conserved from microbes to humans, the same basic mechanisms are in play, as is observed in hyperglycemic memory. Exploration of new metabolic transcriptional memory systems as well as further detailed mechanistic analyses of known memory contexts in microbes is therefore central to understanding metabolic memory in humans, and may be of relevance for the successful treatment of the ever-growing epidemic of diabetes.
Keywords: Bistability; Chromatin modification; Diabetes; Galactose; Glucose; Hyperglycemia; Hysteresis; Inositol; Lactose; Maltose; Metabolic memory; Metabolic network; Protein inheritance; Reinduction memory; Transcriptional memory.
Copyright © 2020 The Author. Published by Elsevier GmbH.. All rights reserved.
Figures


References
-
- Dekel E., Alon U. Optimality and evolutionary tuning of the expression level of a protein. Nature. 2005;436(7050):588–592. - PubMed
-
- Kussell E., Leibler S. Phenotypic diversity, population growth, and information in fluctuating environments. Science. 2005;309(5743):2075–2078. - PubMed
-
- Shoval O., Sheftel H., Shinar G., Hart Y., Ramote O., Mayo A. Evolutionary trade-offs, Pareto optimality, and the geometry of phenotype space. Science. 2012;336(6085):1157–1160. - PubMed
-
- Schuetz R., Zamboni N., Zampieri M., Heinemann M., Sauer U. Multidimensional optimality of microbial metabolism. Science. 2012;336(6081):601–604. - PubMed
-
- Novak M., Pfeiffer T., Lenski R.E., Sauer U., Bonhoeffer S. Experimental tests for an evolutionary trade-off between growth rate and yield in E. coli. The American Naturalist. 2006;168(2):242–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

