Patient barriers to cancer clinical trial participation and navigator activities to assist
- PMID: 32241387
- PMCID: PMC8623462
- DOI: 10.1016/bs.acr.2020.01.008
Patient barriers to cancer clinical trial participation and navigator activities to assist
Abstract
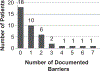
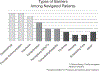
Clinical research is vital to the discovery of new cancer treatments that can enhance health and prolong life for cancer patients, but breakthroughs in cancer treatment are limited by challenges recruiting patients into cancer clinical trials (CT). Only 3-5% of cancer patients in the United States participate in a cancer CT and there are disparities in CT participation by age, race and gender. Strategies such as patient navigation, which is designed to provide patients with education and practical support, may help to overcome challenges of CT recruitment. The current study evaluated an intervention in which lay navigators were utilized to provide patient education and practical support for helping patients overcome barriers to CT participation and related clinical care. A patient barrier checklist was utilized to record patient barriers to CT participation and care, actions taken by navigators to assist patients with these barriers, and whether or not these barriers could be overcome. Forty patients received patient navigation services. The most common barriers faced by navigated patients were fear (n=9), issues communicating with medical personnel (n=9), insurance issues (n=8), transportation difficulties (n=6) and perceptions about providers and treatment (n=4). The most common activities undertaken by navigators were making referrals and contacts on behalf of patients (e.g., support services, family, clinicians; n=25). Navigators also made arrangement for transportation, financial, medication and equipment services for patients (n=11) and proactively navigated patients (n=8). Barriers that were not overcome for two or more patients included insurance issues, lack of temporary housing resources for patients in treatment and assistance with household bills. The wide array of patient barriers to CT participation and navigator assistance documented in this study supports the CT navigator role in facilitating quality care.
Keywords: Barriers to care; Cancer; Clinical trials; Health disparities; Patient navigation; Recruitment; Retention.
© 2020 Elsevier Inc. All rights reserved.
Figures
References
-
- American Cancer Society. (2017). Cancer facts & figures 2017. Atlanta, GA, accessed 12/23/19 at https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts....
-
- BRFSS, & Centers for Disease Control and Prevention (CDC). (2018). Behavioral risk factor surveillance system survey data. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention.
-
- Bryant DC, Williamson D, Cartmell K, & Jefferson M. (2011). A lay patient navigation training curriculum targeting disparities in cancer clinical trials. Journal of National Black Nurses’ Association, 22(2), 68–75. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23061182. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical