The inhibitory effect of phosphorylated Codonopsis pilosula polysaccharide on autophagosomes formation contributes to the inhibition of duck hepatitis A virus replication
- PMID: 32241500
- PMCID: PMC7587719
- DOI: 10.1016/j.psj.2019.11.060
The inhibitory effect of phosphorylated Codonopsis pilosula polysaccharide on autophagosomes formation contributes to the inhibition of duck hepatitis A virus replication
Abstract
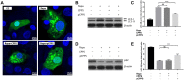
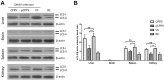
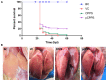
Duck hepatitis A virus type 1 (DHAV) infection causes duck viral hepatitis and results in enormous loss to poultry farming industry. We reported that phosphorylated Codonopsis pilosula polysaccharide (pCPPS) inhibited DHAV genome replication. Here we further explored its underlying antiviral mechanisms. Autophagosomes formation is essential for the genome replication of picornaviruses. In this study, Western blot, confocal microscopy observation, and ELISA methods were performed to analyze polysaccharides' effects on autophagy by the in vitro and in vivo experiments. Results obtained from in vitro and in vivo experiments showed that Codonopsis pilosula polysaccharide did not play a role in regulating autophagy and had no therapeutic effects on infected ducklings. However, pCPPS treatment downregulated LC3-II expression level activated by DHAV and rapamycin, indicating the inhibition of autophagosomes formation. The interdiction of autophagosomes formation resulted in the inhibition of DHAV genome replication. Further study showed that pCPPS treatment reduced the concentration of phosphatidylinositol-3-phosphate (PI3P), an important component of membrane, in cells and serum, and consequently, autophagosomes formation was downregulated. In vivo experiments also verified the therapeutic effect of pCPPS. Phosphorylated Codonopsis pilosula polysaccharide treatment increased the infected ducklings' survival rate and alleviated hepatic injury. Our studies verified the effects of pCPPS against DHAV infection in duck embryo hepatocytes and ducklings and confirmed that phosphorylated modification enhanced the bioactivities of polysaccharides. The results also stated pCPPS's antiviral mechanisms, provided fundamental basis for the development of new anti-DHAV agents.
Keywords: antiviral; autophagy; duck hepatitis A virus; phosphorylated polysaccharide.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Phosphorylated Codonopsis pilosula polysaccharide could inhibit the virulence of duck hepatitis A virus compared with Codonopsis pilosula polysaccharide.Int J Biol Macromol. 2017 Jan;94(Pt A):28-35. doi: 10.1016/j.ijbiomac.2016.10.002. Epub 2016 Oct 3. Int J Biol Macromol. 2017. PMID: 27713010
-
PI3KC3-dependent autophagosomes formation pathway is of crucial importance to anti-DHAV activity of Chrysanthemum indicum polysaccharide.Carbohydr Polym. 2019 Mar 15;208:22-31. doi: 10.1016/j.carbpol.2018.12.035. Epub 2018 Dec 13. Carbohydr Polym. 2019. PMID: 30658794
-
Comparison of the anti-duck hepatitis A virus activities of phosphorylated and sulfated Astragalus polysaccharides.Exp Biol Med (Maywood). 2017 Feb;242(3):344-353. doi: 10.1177/1535370216672750. Epub 2016 Oct 4. Exp Biol Med (Maywood). 2017. PMID: 27703041 Free PMC article.
-
Current status and future direction of duck hepatitis A virus vaccines.Avian Pathol. 2023 Apr;52(2):89-99. doi: 10.1080/03079457.2022.2162367. Epub 2023 Jan 19. Avian Pathol. 2023. PMID: 36571394 Review.
-
Duck hepatitis A virus prevalence in mainland China between 2009 and 2021: A systematic review and meta-analysis.Prev Vet Med. 2022 Nov;208:105730. doi: 10.1016/j.prevetmed.2022.105730. Epub 2022 Jul 30. Prev Vet Med. 2022. PMID: 35964373
Cited by
-
Innovative Approaches to Combat Duck Viral Hepatitis: Dual-Specific Anti-DHAV-1 and DHAV-3 Yolk Antibodies.Vaccines (Basel). 2025 Feb 2;13(2):154. doi: 10.3390/vaccines13020154. Vaccines (Basel). 2025. PMID: 40006701 Free PMC article.
-
Progress of Studies on Plant-Derived Polysaccharides Affecting Intestinal Barrier Function in Poultry.Animals (Basel). 2022 Nov 18;12(22):3205. doi: 10.3390/ani12223205. Animals (Basel). 2022. PMID: 36428432 Free PMC article. Review.
-
A monitoring survey and health risk assessment for pesticide residues on Codonopsis Radix in China.Sci Rep. 2022 May 17;12(1):8133. doi: 10.1038/s41598-022-11428-w. Sci Rep. 2022. PMID: 35581226 Free PMC article.
-
Modulation of cyclophosphamide-induced immunosuppression and intestinal flora in broiler by deep eutectic solvent extracted polysaccharides of Acanthopanax senticosus.Front Vet Sci. 2024 May 27;11:1415716. doi: 10.3389/fvets.2024.1415716. eCollection 2024. Front Vet Sci. 2024. PMID: 38863455 Free PMC article.
-
Immunomodulatory effect of Acanthopanax senticosus polysaccharide on immunosuppressed chickens.Poult Sci. 2021 Feb;100(2):623-630. doi: 10.1016/j.psj.2020.11.059. Epub 2020 Dec 1. Poult Sci. 2021. PMID: 33518115 Free PMC article.
References
-
- Berryman S., Brooks E., Burman A., Hawes P., Roberts R., Netherton C., Monaghan P., Whelband M., Cottam E., Elazar Z., Jackson T., Wileman T. Foot-and-Mouth disease virus induces autophagosomes during cell entry via a class III phosphatidylinositol 3-kinase-independent pathway. J. Virol. 2012;86:12940–12953. - PMC - PubMed
-
- Chen Y., Xiong W., Zeng L., Wang D., Liu J., Wu Y., Hu Y. Comparison of Bush Sophora Root polysaccharide and its sulfate’s anti-duck hepatitis A virus activity and mechanism. Carbohydr. Polym. 2014;102:333–340. - PubMed
-
- Chen Y., Yang Y., Yuan W., Wang Z., Ming K., Zeng L., Liu J. Effects of Bush Sophora Root polysaccharide and its sulfate on DHAV-1 replication. Carbohydr. Polym. 2018;197:508–514. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

