The Neural Origin of Nociceptive-Induced Gamma-Band Oscillations
- PMID: 32241836
- PMCID: PMC7178916
- DOI: 10.1523/JNEUROSCI.0255-20.2020
The Neural Origin of Nociceptive-Induced Gamma-Band Oscillations
Abstract
Gamma-band oscillations (GBOs) elicited by transient nociceptive stimuli are one of the most promising biomarkers of pain across species. Still, whether these GBOs reflect stimulus encoding in the primary somatosensory cortex (S1) or nocifensive behavior in the primary motor cortex (M1) is debated. Here we recorded neural activity simultaneously from the brain surface as well as at different depths of the bilateral S1/M1 in freely-moving male rats receiving nociceptive stimulation. GBOs measured from superficial layers of S1 contralateral to the stimulated paw not only had the largest magnitude, but also showed the strongest temporal and phase coupling with epidural GBOs. Also, spiking of superficial S1 interneurons had the strongest phase coherence with epidural GBOs. These results provide the first direct demonstration that scalp GBOs, one of the most promising pain biomarkers, reflect neural activity strongly coupled with the fast spiking of interneurons in the superficial layers of the S1 contralateral to the stimulated side.SIGNIFICANCE STATEMENT Nociceptive-induced gamma-band oscillations (GBOs) measured at population level are one of the most promising biomarkers of pain perception. Our results provide the direct demonstration that these GBOs reflect neural activity coupled with the spike firing of interneurons in the superficial layers of the primary somatosensory cortex (S1) contralateral to the side of nociceptive stimulation. These results address the ongoing debate about whether nociceptive-induced GBOs recorded with scalp EEG or epidurally reflect stimulus encoding in the S1 or nocifensive behavior in the primary motor cortex (M1), and will therefore influence how experiments in pain neuroscience will be designed and interpreted.
Keywords: biomarkers; gamma-band oscillations; interneurons; pain; primary motor cortex; primary somatosensory cortex.
Copyright © 2020 the authors.
Figures
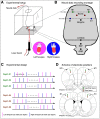
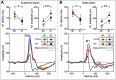
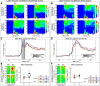



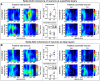
Comment in
-
Intracortical Localization of a Promising Pain Biomarker.J Neurosci. 2020 Dec 9;40(50):9549-9551. doi: 10.1523/JNEUROSCI.1520-20.2020. J Neurosci. 2020. PMID: 33298597 Free PMC article. No abstract available.
References
-
- Adhikari A, Sigurdsson T, Topiwala MA, Gordon JA (2010) Cross-correlation of instantaneous amplitudes of field potential oscillations: a straightforward method to estimate the directionality and lag between brain areas. J Neurosci Methods 191:191–200. 10.1016/j.jneumeth.2010.06.019 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
