Treatment with Mesenchymal-Derived Extracellular Vesicles Reduces Injury-Related Pathology in Pyramidal Neurons of Monkey Perilesional Ventral Premotor Cortex
- PMID: 32241837
- PMCID: PMC7178914
- DOI: 10.1523/JNEUROSCI.2226-19.2020
Treatment with Mesenchymal-Derived Extracellular Vesicles Reduces Injury-Related Pathology in Pyramidal Neurons of Monkey Perilesional Ventral Premotor Cortex
Abstract
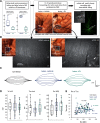
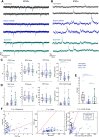
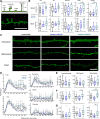
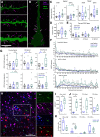
Functional recovery after cortical injury, such as stroke, is associated with neural circuit reorganization, but the underlying mechanisms and efficacy of therapeutic interventions promoting neural plasticity in primates are not well understood. Bone marrow mesenchymal stem cell-derived extracellular vesicles (MSC-EVs), which mediate cell-to-cell inflammatory and trophic signaling, are thought be viable therapeutic targets. We recently showed, in aged female rhesus monkeys, that systemic administration of MSC-EVs enhances recovery of function after injury of the primary motor cortex, likely through enhancing plasticity in perilesional motor and premotor cortices. Here, using in vitro whole-cell patch-clamp recording and intracellular filling in acute slices of ventral premotor cortex (vPMC) from rhesus monkeys (Macaca mulatta) of either sex, we demonstrate that MSC-EVs reduce injury-related physiological and morphologic changes in perilesional layer 3 pyramidal neurons. At 14-16 weeks after injury, vPMC neurons from both vehicle- and EV-treated lesioned monkeys exhibited significant hyperexcitability and predominance of inhibitory synaptic currents, compared with neurons from nonlesioned control brains. However, compared with vehicle-treated monkeys, neurons from EV-treated monkeys showed lower firing rates, greater spike frequency adaptation, and excitatory:inhibitory ratio. Further, EV treatment was associated with greater apical dendritic branching complexity, spine density, and inhibition, indicative of enhanced dendritic plasticity and filtering of signals integrated at the soma. Importantly, the degree of EV-mediated reduction of injury-related pathology in vPMC was significantly correlated with measures of behavioral recovery. These data show that EV treatment dampens injury-related hyperexcitability and restores excitatory:inhibitory balance in vPMC, thereby normalizing activity within cortical networks for motor function.SIGNIFICANCE STATEMENT Neuronal plasticity can facilitate recovery of function after cortical injury, but the underlying mechanisms and efficacy of therapeutic interventions promoting this plasticity in primates are not well understood. Our recent work has shown that intravenous infusions of mesenchymal-derived extracellular vesicles (EVs) that are involved in cell-to-cell inflammatory and trophic signaling can enhance recovery of motor function after injury in monkey primary motor cortex. This study shows that this EV-mediated enhancement of recovery is associated with amelioration of injury-related hyperexcitability and restoration of excitatory-inhibitory balance in perilesional ventral premotor cortex. These findings demonstrate the efficacy of mesenchymal EVs as a therapeutic to reduce injury-related pathologic changes in the physiology and structure of premotor pyramidal neurons and support recovery of function.
Keywords: exosomes; inhibitory neurons; mesenchymal stem cell; motor cortex; neuronal excitability; stroke.
Copyright © 2020 the authors.
Figures



Similar articles
-
Mesenchymal-derived extracellular vesicles enhance microglia-mediated synapse remodeling after cortical injury in aging Rhesus monkeys.J Neuroinflammation. 2023 Sep 2;20(1):201. doi: 10.1186/s12974-023-02880-0. J Neuroinflammation. 2023. PMID: 37660145 Free PMC article.
-
Extracellular vesicles derived from bone marrow mesenchymal stem cells enhance myelin maintenance after cortical injury in aged rhesus monkeys.Exp Neurol. 2021 Mar;337:113540. doi: 10.1016/j.expneurol.2020.113540. Epub 2020 Nov 29. Exp Neurol. 2021. PMID: 33264634 Free PMC article.
-
Neural recovery after cortical injury: Effects of MSC derived extracellular vesicles on motor circuit remodeling in rhesus monkeys.IBRO Neurosci Rep. 2022 Aug 18;13:243-254. doi: 10.1016/j.ibneur.2022.08.001. eCollection 2022 Dec. IBRO Neurosci Rep. 2022. PMID: 36590089 Free PMC article.
-
Training-induced recovery of manual dexterity after a lesion in the motor cortex.Keio J Med. 2010;59(1):4-9. doi: 10.2302/kjm.59.4. Keio J Med. 2010. PMID: 20375652 Review.
-
Integrated technology for evaluation of brain function and neural plasticity.Phys Med Rehabil Clin N Am. 2004 Feb;15(1):263-306. doi: 10.1016/s1047-9651(03)00124-4. Phys Med Rehabil Clin N Am. 2004. PMID: 15029909 Review.
Cited by
-
Reduction of inflammatory biomarkers underlies extracellular vesicle mediated functional recovery in an aged monkey model of cortical injury.Front Aging Neurosci. 2025 Jul 9;17:1605144. doi: 10.3389/fnagi.2025.1605144. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40703679 Free PMC article.
-
Mesenchymal stem cell-derived exosomes: Shaping the next era of stroke treatment.Neuroprotection. 2023 Dec;1(2):99-116. doi: 10.1002/nep3.30. Epub 2023 Dec 30. Neuroprotection. 2023. PMID: 38283953 Free PMC article.
-
Therapeutic role of mesenchymal stem cell-derived extracellular vesicles in neuroinflammation and cognitive dysfunctions induced by binge-like ethanol treatment in adolescent mice.CNS Neurosci Ther. 2023 Dec;29(12):4018-4031. doi: 10.1111/cns.14326. Epub 2023 Jun 28. CNS Neurosci Ther. 2023. PMID: 37381698 Free PMC article.
-
Mesenchymal stem cells and their extracellular vesicle therapy for neurological disorders: traumatic brain injury and beyond.Front Neurol. 2025 Feb 5;16:1472679. doi: 10.3389/fneur.2025.1472679. eCollection 2025. Front Neurol. 2025. PMID: 39974358 Free PMC article. Review.
-
Mesenchymal Stem Cell Application and Its Therapeutic Mechanisms in Intracerebral Hemorrhage.Front Cell Neurosci. 2022 Jun 13;16:898497. doi: 10.3389/fncel.2022.898497. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35769327 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
