Temporal integration of mitogen history in mother cells controls proliferation of daughter cells
- PMID: 32241885
- PMCID: PMC8363187
- DOI: 10.1126/science.aay8241
Temporal integration of mitogen history in mother cells controls proliferation of daughter cells
Abstract
Multicellular organisms use mitogens to regulate cell proliferation, but how fluctuating mitogenic signals are converted into proliferation-quiescence decisions is poorly understood. In this work, we combined live-cell imaging with temporally controlled perturbations to determine the time scale and mechanisms underlying this system in human cells. Contrary to the textbook model that cells sense mitogen availability only in the G1 cell cycle phase, we find that mitogenic signaling is temporally integrated throughout the entire mother cell cycle and that even a 1-hour lapse in mitogen signaling can influence cell proliferation more than 12 hours later. Protein translation rates serve as the integrator that proportionally converts mitogen history into corresponding levels of cyclin D in the G2 phase of the mother cell, which controls the proliferation-quiescence decision in daughter cells and thereby couples protein production with cell proliferation.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

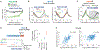
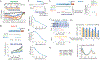
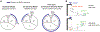
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

