Polarization of protease-activated receptor 2 (PAR-2) signaling is altered during airway epithelial remodeling and deciliation
- PMID: 32241907
- PMCID: PMC7212650
- DOI: 10.1074/jbc.RA120.012710
Polarization of protease-activated receptor 2 (PAR-2) signaling is altered during airway epithelial remodeling and deciliation
Abstract
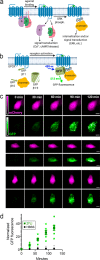
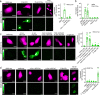
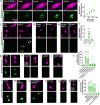
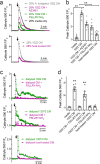
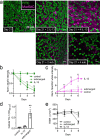
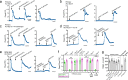
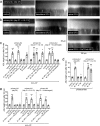
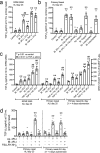
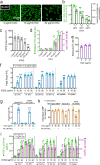
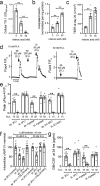
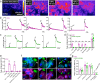
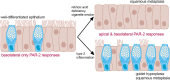
Protease-activated receptor 2 (PAR-2) is activated by secreted proteases from immune cells or fungi. PAR-2 is normally expressed basolaterally in differentiated nasal ciliated cells. We hypothesized that epithelial remodeling during diseases characterized by cilial loss and squamous metaplasia may alter PAR-2 polarization. Here, using a fluorescent arrestin assay, we confirmed that the common fungal airway pathogen Aspergillus fumigatus activates heterologously-expressed PAR-2. Endogenous PAR-2 activation in submerged airway RPMI 2650 or NCI-H520 squamous cells increased intracellular calcium levels and granulocyte macrophage-colony-stimulating factor, tumor necrosis factor α, and interleukin (IL)-6 secretion. RPMI 2650 cells cultured at an air-liquid interface (ALI) responded to apically or basolaterally applied PAR-2 agonists. However, well-differentiated primary nasal epithelial ALIs responded only to basolateral PAR-2 stimulation, indicated by calcium elevation, increased cilia beat frequency, and increased fluid and cytokine secretion. We exposed primary cells to disease-related modifiers that alter epithelial morphology, including IL-13, cigarette smoke condensate, and retinoic acid deficiency, at concentrations and times that altered epithelial morphology without causing breakdown of the epithelial barrier to model early disease states. These altered primary cultures responded to both apical and basolateral PAR-2 stimulation. Imaging nasal polyps and control middle turbinate explants, we found that nasal polyps, but not turbinates, exhibit apical calcium responses to PAR-2 stimulation. However, isolated ciliated cells from both polyps and turbinates maintained basolateral PAR-2 polarization, suggesting that the calcium responses originated from nonciliated cells. Altered PAR-2 polarization in disease-remodeled epithelia may enhance apical responses and increase sensitivity to inhaled proteases.
Keywords: Aspergillus; G-protein–coupled receptor (GPCR); calcium; chloride transport; cilia; cytokine; inflammation; mucociliary clearance; mucosal immunology; protease.
© 2020 Carey et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Protease-activated receptor 2 activates airway apical membrane chloride permeability and increases ciliary beating.FASEB J. 2018 Jan;32(1):155-167. doi: 10.1096/fj.201700114RRR. Epub 2017 Sep 5. FASEB J. 2018. PMID: 28874459 Free PMC article.
-
Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2.Int Arch Allergy Immunol. 2012;158 Suppl 1(Suppl 1):19-29. doi: 10.1159/000337756. Epub 2012 May 15. Int Arch Allergy Immunol. 2012. PMID: 22627362 Free PMC article.
-
A highly potent agonist to protease-activated receptor-2 reveals apical activation of the airway epithelium resulting in Ca2+-regulated ion conductance.Am J Physiol Cell Physiol. 2014 Oct 15;307(8):C718-26. doi: 10.1152/ajpcell.00257.2014. Am J Physiol Cell Physiol. 2014. PMID: 25143347 Free PMC article.
-
The role of protease activation of inflammation in allergic respiratory diseases.J Allergy Clin Immunol. 2004 Nov;114(5):997-1008; quiz 1009. doi: 10.1016/j.jaci.2004.07.060. J Allergy Clin Immunol. 2004. PMID: 15536399 Review.
-
Bronchial epithelium as a target for innovative treatments in asthma.Pharmacol Ther. 2013 Dec;140(3):290-305. doi: 10.1016/j.pharmthera.2013.07.008. Epub 2013 Jul 21. Pharmacol Ther. 2013. PMID: 23880290 Review.
Cited by
-
PAR-2-activated secretion by airway gland serous cells: role for CFTR and inhibition by Pseudomonas aeruginosa.Am J Physiol Lung Cell Mol Physiol. 2021 May 1;320(5):L845-L879. doi: 10.1152/ajplung.00411.2020. Epub 2021 Mar 3. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33655758 Free PMC article.
-
Extracellular Nucleotides and Histamine Suppress TLR3- and RIG-I-Mediated Release of Antiviral IFNs from Human Airway Epithelial Cells.J Immunol. 2022 May 15;208(10):2390-2402. doi: 10.4049/jimmunol.2101085. Epub 2022 Apr 22. J Immunol. 2022. PMID: 35459743 Free PMC article.
-
T2R bitter taste receptors regulate apoptosis and may be associated with survival in head and neck squamous cell carcinoma.Mol Oncol. 2022 Apr;16(7):1474-1492. doi: 10.1002/1878-0261.13131. Epub 2021 Dec 14. Mol Oncol. 2022. PMID: 34717036 Free PMC article.
-
Effects of Akt Activator SC79 on Human M0 Macrophage Phagocytosis and Cytokine Production.Cells. 2024 May 24;13(11):902. doi: 10.3390/cells13110902. Cells. 2024. PMID: 38891035 Free PMC article.
-
Cilia Stimulatory and Antibacterial Activities of T2R Bitter Taste Receptor Agonist Diphenhydramine: Insights into Repurposing Bitter Drugs for Nasal Infections.Pharmaceuticals (Basel). 2022 Apr 6;15(4):452. doi: 10.3390/ph15040452. Pharmaceuticals (Basel). 2022. PMID: 35455449 Free PMC article.
References
-
- Matsuwaki Y., Wada K., White T., Moriyama H., and Kita H. (2012) Alternaria fungus induces the production of GM-CSF, interleukin-6, and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2. Int. Arch. Allergy Immunol. 158, Suppl. 1, 19–29 10.1159/000337756 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

