Biosynthesis of the modified tetrapyrroles-the pigments of life
- PMID: 32241908
- PMCID: PMC7242693
- DOI: 10.1074/jbc.REV120.006194
Biosynthesis of the modified tetrapyrroles-the pigments of life
Abstract
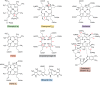
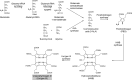
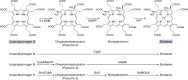
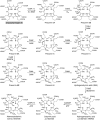
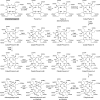
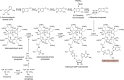
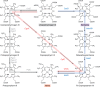
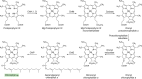
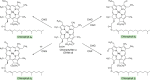
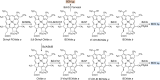
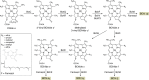
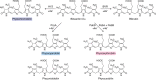
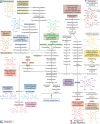
Modified tetrapyrroles are large macrocyclic compounds, consisting of diverse conjugation and metal chelation systems and imparting an array of colors to the biological structures that contain them. Tetrapyrroles represent some of the most complex small molecules synthesized by cells and are involved in many essential processes that are fundamental to life on Earth, including photosynthesis, respiration, and catalysis. These molecules are all derived from a common template through a series of enzyme-mediated transformations that alter the oxidation state of the macrocycle and also modify its size, its side-chain composition, and the nature of the centrally chelated metal ion. The different modified tetrapyrroles include chlorophylls, hemes, siroheme, corrins (including vitamin B12), coenzyme F430, heme d1, and bilins. After nearly a century of study, almost all of the more than 90 different enzymes that synthesize this family of compounds are now known, and expression of reconstructed operons in heterologous hosts has confirmed that most pathways are complete. Aside from the highly diverse nature of the chemical reactions catalyzed, an interesting aspect of comparative biochemistry is to see how different enzymes and even entire pathways have evolved to perform alternative chemical reactions to produce the same end products in the presence and absence of oxygen. Although there is still much to learn, our current understanding of tetrapyrrole biogenesis represents a remarkable biochemical milestone that is summarized in this review.
Keywords: 5-aminolevulinic acid; adenosylcobalamin (AdoCbl); bacteriochlorophyll; bilin; biosynthesis; chlorophyll; cobalamin; coenzyme F430; heme; heme d1; photosynthesis; precorrin; tetrapyrrole; uroporphyrinogen III; vitamin B12.
© 2020 Bryant et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

