Cysteine depletion induces pancreatic tumor ferroptosis in mice
- PMID: 32241947
- PMCID: PMC7681911
- DOI: 10.1126/science.aaw9872
Cysteine depletion induces pancreatic tumor ferroptosis in mice
Abstract
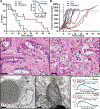
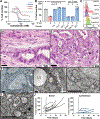
Ferroptosis is a form of cell death that results from the catastrophic accumulation of lipid reactive oxygen species (ROS). Oncogenic signaling elevates lipid ROS production in many tumor types and is counteracted by metabolites that are derived from the amino acid cysteine. In this work, we show that the import of oxidized cysteine (cystine) via system xC - is a critical dependency of pancreatic ductal adenocarcinoma (PDAC), which is a leading cause of cancer mortality. PDAC cells used cysteine to synthesize glutathione and coenzyme A, which, together, down-regulated ferroptosis. Studying genetically engineered mice, we found that the deletion of a system xC - subunit, Slc7a11, induced tumor-selective ferroptosis and inhibited PDAC growth. This was replicated through the administration of cyst(e)inase, a drug that depletes cysteine and cystine, demonstrating a translatable means to induce ferroptosis in PDAC.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


References
-
- Sato H et al. , Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem 280, 37423–37429 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

