The regulatory network among CircHIPK3, LncGAS5, and miR-495 promotes Th2 differentiation in allergic rhinitis
- PMID: 32242002
- PMCID: PMC7118158
- DOI: 10.1038/s41419-020-2394-3
The regulatory network among CircHIPK3, LncGAS5, and miR-495 promotes Th2 differentiation in allergic rhinitis
Abstract
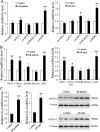
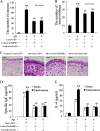
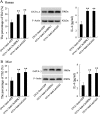
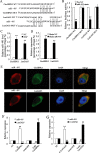
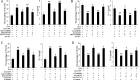
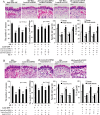
Allergic rhinitis (AR) is a common allergic disease which is characterized by the promotion of Th2 differentiation of CD4+ T cells. However, the mechanisms underlying Th2 differentiation remain unclear. Non-coding RNAs play a critical role in Th2 differentiation, whereas few studies have revealed the interactions among long non-coding RNAs, circular RNAs, and microRNAs. In this study, the differential expressions of several circRNAs and lncRNAs were compared in nasal mucosa samples of AR patients and mice with experimentally induced AR as compared to healthy controls. The results showed that the highly expressed CircHIPK3 and LncGAS5 promoted Th2 differentiation of ovalbumin-induced CD4+ T cells and aggravated nasal symptoms of AR mice. We also found that CircHIPK3 and LncGAS5 induced the upregulation of Th2 cell-specific transcript factor GATA-3 via modulating their common target miR-495. Meanwhile, the intranasal administration of CircHIPK3 or LncGAS5 knockdown lentivirus decreased nasal symptoms of AR mice. In conclusion, our findings indicated that the interactions among CircHIPK3, LncGAS5, and miR-495 play a critical role in the regulation of Th2 differentiation in AR.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Long non-coding RNA MALAT1 promotes Th2 differentiation by regulating microRNA-135b-5p/GATA-3 axis in children with allergic rhinitis.Kaohsiung J Med Sci. 2022 Oct;38(10):971-980. doi: 10.1002/kjm2.12587. Epub 2022 Sep 23. Kaohsiung J Med Sci. 2022. PMID: 36149748 Free PMC article.
-
Exosomal long non-coding RNA GAS5 suppresses Th1 differentiation and promotes Th2 differentiation via downregulating EZH2 and T-bet in allergic rhinitis.Mol Immunol. 2020 Feb;118:30-39. doi: 10.1016/j.molimm.2019.11.009. Epub 2019 Dec 13. Mol Immunol. 2020. PMID: 31841965
-
Mesenchymal Stem Cell-Derived Exosome-Containing Linc00632 Regulates GATA Binding Protein-3 Expression in Allergic Rhinitis by Interacting with Enhancer of Zeste Homolog 2 to Inhibit T Helper Cell 2 Differentiation.Int Arch Allergy Immunol. 2022;183(2):235-245. doi: 10.1159/000518950. Epub 2021 Sep 17. Int Arch Allergy Immunol. 2022. PMID: 34537772
-
Roles of noncoding RNA in allergic rhinitis.Int Forum Allergy Rhinol. 2024 Nov;14(11):1757-1775. doi: 10.1002/alr.23461. Epub 2024 Oct 5. Int Forum Allergy Rhinol. 2024. PMID: 39367803 Review.
-
Role of non-coding RNAs as biomarkers of deleterious cardiovascular effects in sepsis.Prog Cardiovasc Dis. 2021 Sep-Oct;68:70-77. doi: 10.1016/j.pcad.2021.07.005. Epub 2021 Jul 13. Prog Cardiovasc Dis. 2021. PMID: 34265333 Review.
Cited by
-
[Research progress on the relationship between lncRNA and the pathogenesis of allergic rhinitis].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2022 Mar;36(3):233-238. doi: 10.13201/j.issn.2096-7993.2022.03.016. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2022. PMID: 35193349 Free PMC article. Review. Chinese.
-
Hsa_circRNA_100791 Modulates Trim13 Through Sponging miR-487b-5p to Facilitate Inflammation in Allergic Rhinitis.J Inflamm Res. 2024 Dec 17;17:11175-11193. doi: 10.2147/JIR.S485165. eCollection 2024. J Inflamm Res. 2024. PMID: 39713717 Free PMC article.
-
The role of miRNAs in T helper cell development, activation, fate decisions and tumor immunity.Front Immunol. 2024 Jan 9;14:1320305. doi: 10.3389/fimmu.2023.1320305. eCollection 2023. Front Immunol. 2024. PMID: 38264670 Free PMC article. Review.
-
Emerging Role of Non-Coding RNAs in Regulation of T-Lymphocyte Function.Front Immunol. 2021 Nov 4;12:756042. doi: 10.3389/fimmu.2021.756042. eCollection 2021. Front Immunol. 2021. PMID: 34804042 Free PMC article. Review.
-
Non-coding RNAs in immunoregulation and autoimmunity: Technological advances and critical limitations.J Autoimmun. 2023 Jan;134:102982. doi: 10.1016/j.jaut.2022.102982. Epub 2022 Dec 31. J Autoimmun. 2023. PMID: 36592512 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

