GPR56/ADGRG1 is associated with response to antidepressant treatment
- PMID: 32242018
- PMCID: PMC7118175
- DOI: 10.1038/s41467-020-15423-5
GPR56/ADGRG1 is associated with response to antidepressant treatment
Abstract
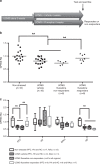
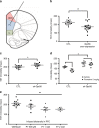
It remains unclear why many patients with depression do not respond to antidepressant treatment. In three cohorts of individuals with depression and treated with serotonin-norepinephrine reuptake inhibitor (N = 424) we show that responders, but not non-responders, display an increase of GPR56 mRNA in the blood. In a small group of subjects we also show that GPR56 is downregulated in the PFC of individuals with depression that died by suicide. In mice, we show that chronic stress-induced Gpr56 downregulation in the blood and prefrontal cortex (PFC), which is accompanied by depression-like behavior, and can be reversed by antidepressant treatment. Gpr56 knockdown in mouse PFC is associated with depressive-like behaviors, executive dysfunction and poor response to antidepressant treatment. GPR56 peptide agonists have antidepressant-like effects and upregulated AKT/GSK3/EIF4 pathways. Our findings uncover a potential role of GPR56 in antidepressant response.
Trial registration: ClinicalTrials.gov NCT00635219 NCT00599911 NCT01140906 NCT02209142.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- WHO. Depression and Other Common Mental Disorders: Global Health Estimates (2017).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

