Biomarker Exploration in Human Peripheral Blood Mononuclear Cells for Monitoring Sulforaphane Treatment Responses in Autism Spectrum Disorder
- PMID: 32242086
- PMCID: PMC7118069
- DOI: 10.1038/s41598-020-62714-4
Biomarker Exploration in Human Peripheral Blood Mononuclear Cells for Monitoring Sulforaphane Treatment Responses in Autism Spectrum Disorder
Abstract
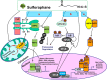
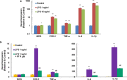
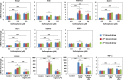
Autism Spectrum Disorder (ASD) is one of the most common neurodevelopmental disorders with no drugs treating the core symptoms and no validated biomarkers for clinical use. The multi-functional phytochemical sulforaphane affects many of the biochemical abnormalities associated with ASD. We investigated potential molecular markers from three ASD-associated physiological pathways that can be affected by sulforaphane: redox metabolism/oxidative stress; heat shock response; and immune dysregulation/inflammation, in peripheral blood mononuclear cells (PBMCs) from healthy donors and patients with ASD. We first analyzed the mRNA levels of selected molecular markers in response to sulforaphane ex vivo treatment in PBMCs from healthy donors by real-time quantitative PCR. All of the tested markers showed quantifiability, accuracy and reproducibility. We then compared the expression levels of those markers in PBMCs taken from ASD patients in response to orally-delivered sulforaphane. The mRNA levels of cytoprotective enzymes (NQO1, HO-1, AKR1C1), and heat shock proteins (HSP27 and HSP70), increased. Conversely, mRNA levels of pro-inflammatory markers (IL-6, IL-1β, COX-2 and TNF-α) decreased. Individually none is sufficiently specific or sensitive, but when grouped by function as two panels, these biomarkers show promise for monitoring pharmacodynamic responses to sulforaphane in both healthy and autistic humans, and providing guidance for biomedical interventions.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

