Nemo-like kinase reduces mutant huntingtin levels and mitigates Huntington's disease
- PMID: 32242231
- PMCID: PMC7254850
- DOI: 10.1093/hmg/ddaa061
Nemo-like kinase reduces mutant huntingtin levels and mitigates Huntington's disease
Abstract
Nemo-like kinase (NLK), an evolutionarily conserved serine/threonine kinase, is highly expressed in the brain, but its function in the adult brain remains not well understood. In this study, we identify NLK as an interactor of huntingtin protein (HTT). We report that NLK levels are significantly decreased in HD human brain and HD models. Importantly, overexpression of NLK in the striatum attenuates brain atrophy, preserves striatal DARPP32 levels and reduces mutant HTT (mHTT) aggregation in HD mice. In contrast, genetic reduction of NLK exacerbates brain atrophy and loss of DARPP32 in HD mice. Moreover, we demonstrate that NLK lowers mHTT levels in a kinase activity-dependent manner, while having no significant effect on normal HTT protein levels in mouse striatal cells, human cells and HD mouse models. The NLK-mediated lowering of mHTT is associated with enhanced phosphorylation of mHTT. Phosphorylation defective mutation of serine at amino acid 120 (S120) abolishes the mHTT-lowering effect of NLK, suggesting that S120 phosphorylation is an important step in the NLK-mediated lowering of mHTT. A further mechanistic study suggests that NLK promotes mHTT ubiquitination and degradation via the proteasome pathway. Taken together, our results indicate a protective role of NLK in HD and reveal a new molecular target to reduce mHTT levels.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

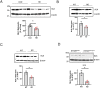
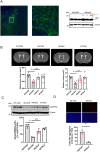

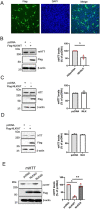

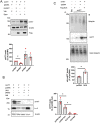
References
-
- The Huntington's Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell, 72, 971–983. - PubMed
-
- Lu, B. and Palacino, J. (2013) A novel human embryonic stem cell-derived Huntington's disease neuronal model exhibits mutant huntingtin (mHTT) aggregates and soluble mHTT-dependent neurodegeneration. FASEB J., 27, 1820–1829. - PubMed
-
- Wanker, E.E., Ast, A., Schindler, F., Trepte, P. and Schnoegl, S. (2019) The pathobiology of perturbed mutant huntingtin protein-protein interactions in Huntington's disease. J. Neurochem., 151, 507–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

