Differential Effects of Nicotine and Nicotine Withdrawal on Fear Conditioning in Male Rats
- PMID: 32242615
- PMCID: PMC7387768
- DOI: 10.1093/ijnp/pyaa024
Differential Effects of Nicotine and Nicotine Withdrawal on Fear Conditioning in Male Rats
Abstract
Background: Tobacco use is prevalent in individuals who are routinely exposed to stress. However, little is known about how nicotine affects responses to trauma. We examined in rats how nicotine exposure affects fear conditioning, a procedure often used to study stress-related psychiatric illness.
Methods: We examined 2 methods of nicotine exposure: self-administration, modeling voluntary use, and experimenter-programmed subcutaneous administration, modeling medicinal administration (nicotine patch). For self-administered nicotine, rats trained to self-administer nicotine i.v. were fear conditioned (via light cue preceding foot-shock) either immediately after a 12-hour self-administration session or 12 hours later during a period with somatic signs of nicotine withdrawal. For experimenter-delivered nicotine, rats were conditioned after 1-21 days of nicotine delivered by programmable (12 hours on) subcutaneous mini-pumps. Tests to evaluate acoustic startle responses to the conditioning environment (context-potentiated startle) and in the presence or absence of the light cue (fear-potentiated startle) occurred after a 10-day period.
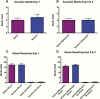
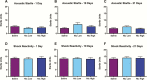
Results: Rats fear conditioned immediately after nicotine self-administration showed reduced responses to the shock-associated context, whereas those trained during nicotine withdrawal showed exaggerated responses. Experimenter-programmed nicotine produced effects qualitatively similar to those seen with self-administered nicotine.
Conclusions: Self-administration or experimenter-programmed delivery of nicotine immediately before exposure to aversive events can reduce conditioned fear responses. In contrast, exposure to aversive events during nicotine withdrawal exacerbates fear responses. These studies raise the possibility of developing safe and effective methods to deliver nicotine or related drugs to mitigate the effects of stress while also highlighting the importance of preventing withdrawal in nicotine-dependent individuals.
Keywords: Fear conditioning; PTSD; nicotine; withdrawal.
© The Author(s) 2020. Published by Oxford University Press on behalf of CINP.
Figures





References
-
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association.
-
- Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Evins AE(2008) The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology 33:480–490. - PubMed
-
- Bauco P, Wise RA (1994) Potentiation of lateral hypothalamic and midline mesencephalic brain stimulation reinforcement by nicotine: examination of repeated treatment. J Pharmacol Exp Ther 271:294–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

