Laboratory preparedness for SARS-CoV-2 testing in India: Harnessing a network of Virus Research & Diagnostic Laboratories
- PMID: 32242875
- PMCID: PMC7258754
- DOI: 10.4103/ijmr.IJMR_594_20
Laboratory preparedness for SARS-CoV-2 testing in India: Harnessing a network of Virus Research & Diagnostic Laboratories
Abstract
Background & objectives: An outbreak of respiratory illness of unknown aetiology was reported from Hubei province of Wuhan, People's Republic of China, in December 2019. The outbreak was attributed to a novel coronavirus (CoV), named as severe acute respiratory syndrome (SARS)-CoV-2 and the disease as COVID-19. Within one month, cases were reported from 25 countries. In view of the novel viral strain with reported high morbidity, establishing early countrywide diagnosis to detect imported cases became critical. Here we describe the role of a countrywide network of VRDLs in early diagnosis of COVID-19.
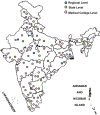
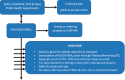
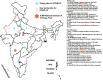
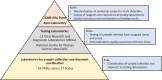
Methods: The Indian Council of Medical Research (ICMR)-National Institute of Virology (NIV), Pune, established screening as well as confirmatory assays for SARS-CoV-2. A total of 13 VRDLs were provided with the E gene screening real-time reverse transcription-polymerase chain reaction (rRT-PCR) assay. VRDLs were selected on the basis of their presence near an international airport/seaport and their past performance. The case definition for testing included all individuals with travel history to Wuhan and symptomatic individuals with travel history to other parts of China. This was later expanded to include symptomatic individuals returning from Singapore, Japan, Hong Kong, Thailand and South Korea.
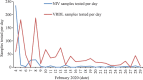
Results: Within a week of standardization of the test at NIV, all VRDLs could initiate testing for SARS-CoV-2. Till February 29, 2020, a total of 2,913 samples were tested. This included both 654 individuals quarantined in the two camps and others fitting within the case definition. The quarantined individuals were tested twice - at days 0 and 14. All tested negative on both occasions. Only three individuals belonging to different districts in Kerala were found to be positive.
Interpretation & conclusions: Sudden emergence of SARS-CoV-2 and its potential to cause a pandemic posed an unsurmountable challenge to the public health system of India. However, concerted efforts of various arms of the Government of India resulted in a well-coordinated action at each level. India has successfully demonstrated its ability to establish quick diagnosis of SARS-CoV-2 at NIV, Pune, and the testing VRDLs.
Keywords: Virus Research and Diagnostic Laboratory; diagnosis; preparedness; quality control; quarantine; severe acute respiratory syndrome-CoV-2; COVID-19.
Conflict of interest statement
None
Figures
Comment in
-
Pandemic SARS-CoV-2 laboratory preparedness in India: An opportunity beyond diagnostics.Indian J Med Res. 2020 May;151(5):495-496. doi: 10.4103/ijmr.IJMR_1548_20. Indian J Med Res. 2020. PMID: 32611920 Free PMC article. No abstract available.
-
Authors' response.Indian J Med Res. 2020 May;151(5):496. doi: 10.4103/0971-5916.286255. Indian J Med Res. 2020. PMID: 32611921 Free PMC article. No abstract available.
References
-
- Knipe DM, Howley PM, editors. Fields virology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2013.
-
- World Health Organization. Coronavirus. WHO; 2020. [accessed on January 21, 2020]. Available from: https://wwwwhoint/health-topics/coronavirus .
-
- World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. [accessed on February 29, 2020]. Available from: https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/technical-... .
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
