Development of in vitro transcribed RNA as positive control for laboratory diagnosis of SARS-CoV-2 in India
- PMID: 32242876
- PMCID: PMC7224626
- DOI: 10.4103/ijmr.IJMR_671_20
Development of in vitro transcribed RNA as positive control for laboratory diagnosis of SARS-CoV-2 in India
Conflict of interest statement
None
Figures
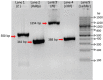

Comment on
-
Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR.Euro Surveill. 2020 Jan;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. Euro Surveill. 2020. PMID: 31992387 Free PMC article.
References
-
- Department of Health Research, Ministry of Health & Family Welfare, Government of India. Establishment of a network of laboratories for managing epidemics and natural calamities (VRDL) [accessed on February 29, 2020]. Available from: https://dhr.gov.in/schemes/esablishment-network-laboratories-managing-ep... .
-
- World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. WHO; 2020. [accessed on January 17, 2020]. Available from: https://wwwwhoint/publications-detail/laboratory-testing-for-2019-novel-... .
-
- World Health Organization. Coronavirus disease (COVID-19) technical guidance: Laboratory testing for 2019-nCoV in humans. WHO; 2020. [accessed on January 17, 2020]. Available from: https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/technical-... .
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
