Reduced neurosteroid potentiation of GABAA receptors in epilepsy and depolarized hippocampal neurons
- PMID: 32243088
- PMCID: PMC7187710
- DOI: 10.1002/acn3.51023
Reduced neurosteroid potentiation of GABAA receptors in epilepsy and depolarized hippocampal neurons
Abstract
Objective: Neurosteroids regulate neuronal excitability by potentiating γ-aminobutyric acid type-A receptors (GABARs). In animal models of temporal lobe epilepsy, the neurosteroid sensitivity of GABARs is diminished and GABAR subunit composition is altered. We tested whether similar changes occur in patients with epilepsy and if depolarization-induced increases in neuronal activity can replicate this effect.
Methods: We determined GABAR α4 subunit expression in cortical tissue resected from pediatric epilepsy patients. Modulation of human GABARs by allopregnanolone and Ro15-4513 was measured in Xenopus oocytes using whole-cell patch clamp. To extend the findings obtained using tissue from epilepsy patients, we evaluated GABAR expression and modulation by allopregnanolone and Ro15-4513 in cultured rat hippocampal neurons exposed to high extracellular potassium (HK) to increase neuronal activity.
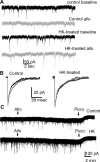
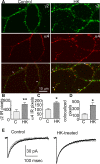
Results: Expression of α4 subunits was increased in pediatric cortical epilepsy specimens encompassing multiple pathologies. The potentiation of GABA-evoked currents by the neurosteroid allopregnanolone was decreased in Xenopus oocytes expressing GABARs isolated from epilepsy patients. Furthermore, receptors isolated from epilepsy but not control tissue were sensitive to potentiation by Ro15-4513, indicating higher expression of α4 βx γ2 subunit-containing receptors. Correspondingly, increasing the activity of cultured rat hippocampal neurons reduced allopregnanolone potentiation of miniature inhibitory postsynaptic currents (mIPSCs), increased modulation of tonic GABAR current by Ro15-4513, upregulated the surface expression of α4 and γ2 subunits, and increased the colocalization of α4 and γ2 subunit immunoreactivity.
Interpretation: These findings suggest that seizure activity-induced upregulation of α4 βx γ2 subunit-containing GABARs could affect the anticonvulsant actions of neurosteroids.
© 2020 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Conflict of interest statement
The authors do not have any conflict of interest.
Figures




References
-
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci 2005;6:565–575. - PubMed
-
- Joshi S, Kapur J. Neurosteroid regulation of seizures: role of GABAA receptor plasticity In: Talevi A., Rocha L., eds. Antiepileptic drug discovery: novel approaches. 1st ed. pp. 127–46. New York, NY: Springer, 2016.
-
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with GABA‐evoked chloride current potentiation. J Pharmacol Exp Ther 1994;270:1223–1229. - PubMed
-
- Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol 2003;53:390–391. - PubMed
-
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia 2001;42:328–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

