Surface Tethering of Inflammation-Modulatory Nanostimulators to Stem Cells for Ischemic Muscle Repair
- PMID: 32243129
- PMCID: PMC8274413
- DOI: 10.1021/acsnano.9b04926
Surface Tethering of Inflammation-Modulatory Nanostimulators to Stem Cells for Ischemic Muscle Repair
Abstract
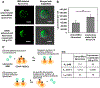
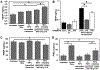
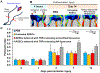
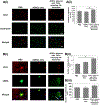
Stem cell transplantation has been a promising treatment for peripheral arterial diseases in the past decade. Stem cells act as living bioreactors of paracrine factors that orchestrate tissue regeneration. Prestimulated adipose-derived stem cells (ADSCs) have been proposed as potential candidates but have been met with challenges in activating their secretory activities for clinical use. Here, we propose that tethering the ADSC surface with nanoparticles releasing tumor necrosis factor α (TNFα), named nanostimulator, would stimulate cellular secretory activity in situ. We examined this hypothesis by complexing octadecylamine-grafted hyaluronic acid onto a liposomal carrier of TNFα. Hyaluronic acid increased the liposomal stability and association to CD44 on ADSC surface. ADSCs tethered with these TNFα carriers exhibited up-regulated secretion of proangiogenic vascular endothelial growth factor and immunomodulatory prosteoglandin E2 (PGE2) while decreasing secretion of antiangiogenic pigment epithelium-derived factors. Accordingly, ADSCs tethered with nanostimulators promoted vascularization in a 3D microvascular chip and enhanced recovery of perfusion, walking, and muscle mass in a murine ischemic hindlimb compared to untreated ADSCs. We propose that this surface tethering strategy for in situ stimulation of stem cells would replace the costly and cumbersome preconditioning process and expedite clinical use of stem cells for improved treatments of various injuries and diseases.
Keywords: adipose-derived stem cells; angiogenesis; hyaluronic acid; liposome; muscle; vascular endothelial growth factor.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Ito WD; Arras M; Winkler B; Scholz D; Schaper J; Schaper W Monocyte Chemotactic Protein-1 Increases Collateral and Peripheral Conductance after Femoral Artery Occlusion. Circ. Res 1997, 80, 829–837. - PubMed
-
- Spaggiari GM; Abdelrazik H; Becchetti F; Moretta L MSCs Inhibit Monocyte-Derived DC Maturation and Function by Selectively Interfering with the Generation of Immature DCs: Central Role of MSC-Derived Prostaglandin E2. Blood 2009, 113, 6576–6583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

