Current and Future Roles of Artificial Intelligence in Medicinal Chemistry Synthesis
- PMID: 32243158
- PMCID: PMC7457232
- DOI: 10.1021/acs.jmedchem.9b02120
Current and Future Roles of Artificial Intelligence in Medicinal Chemistry Synthesis
Abstract
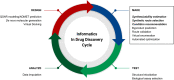
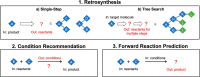
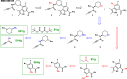
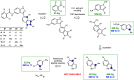
Artificial intelligence and machine learning have demonstrated their potential role in predictive chemistry and synthetic planning of small molecules; there are at least a few reports of companies employing in silico synthetic planning into their overall approach to accessing target molecules. A data-driven synthesis planning program is one component being developed and evaluated by the Machine Learning for Pharmaceutical Discovery and Synthesis (MLPDS) consortium, comprising MIT and 13 chemical and pharmaceutical company members. Together, we wrote this perspective to share how we think predictive models can be integrated into medicinal chemistry synthesis workflows, how they are currently used within MLPDS member companies, and the outlook for this field.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Murcko M. A. Envisioning the Future: Medicine in the Year 2050. Disruptive Sci. Technol. 2012, 1 (2), 89–99. 10.1089/dst.2012.0008. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

