2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors
- PMID: 32243307
- PMCID: PMC7147428
- DOI: 10.1097/LGT.0000000000000525
2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors
Erratum in
-
2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors: Erratum.J Low Genit Tract Dis. 2020 Oct;24(4):427. doi: 10.1097/LGT.0000000000000563. J Low Genit Tract Dis. 2020. PMID: 32732649 No abstract available.
Conflict of interest statement
The National Cancer Institute (including M.S. and N.W.) receives cervical screening results at reduced or no cost from commercial research partners (Qiagen, Roche, BD, MobileODT, Arbor Vita) for independent evaluations of screening methods and strategies. A.-B.M. is an advisory board member of Merck and GSK. R.S.G. is an ASCCP consultant of Inovio Pharmaceuticals DSMB. W.K.H. is connected with Inovio Pharmaceuticals DSMB. P.E.C. has received HPV tests and assays at a reduced or no cost from Roche, Becton Dickinson, Arbor Vita Corporation, and Cepheid for research. M.H.E. has advised companies and participated in educational activities but does not receive any honoraria or payments for these activities, In some cases, his employer, Rutgers, receives payment for his time for these activities from Papivax, Cynvec, Merck, Hologic, and PDS Biotechnologies. He has been the overall PI or local PI for clinical trials from Johnson&Johnson, Pfizer, Iovance, and Inovio. Funding for these activities is for the research related costs of the trials. The other authors have declared they have no conflicts of interest.
Figures

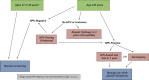
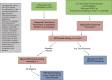
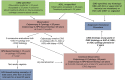
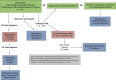
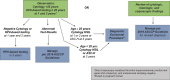

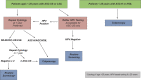
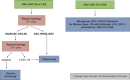
Comment in
-
Response to Letter to the Editor Regarding: 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors.J Low Genit Tract Dis. 2020 Oct;24(4):426. doi: 10.1097/LGT.0000000000000562. J Low Genit Tract Dis. 2020. PMID: 32732648 No abstract available.
-
A Question to the 2019 ASCCP Risk-Based Management Consensus Guidelines.J Low Genit Tract Dis. 2020 Oct;24(4):425. doi: 10.1097/LGT.0000000000000561. J Low Genit Tract Dis. 2020. PMID: 32796263 No abstract available.
References
-
- Wright TC, Cox JT, Massad LS, et al. 2001 Consensus Guidelines for the Management of Women with Cervical Cytological Abnormalities. J Low Genit Tract Dis 2002;6:127–43. - PubMed
-
- Wright TC, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol 2007;197:346–55. - PubMed
-
- Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013;121:829–46. - PubMed
-
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol 2015;136:178–82. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

