A Systematic Review of Tests for Postcolposcopy and Posttreatment Surveillance
- PMID: 32243310
- PMCID: PMC7141755
- DOI: 10.1097/LGT.0000000000000526
A Systematic Review of Tests for Postcolposcopy and Posttreatment Surveillance
Abstract
Objective: For the 2019 ASCCP Risk-Based Management Consensus Guidelines, we conducted a systematic review of diagnostic assays for postcolposcopy and posttreatment management.
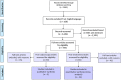
Materials and methods: A literature search was conducted to identify articles reporting on tests/assays for cervical cancer screening, triage, postcolposcopy surveillance, and posttreatment surveillance published between 2012 and 2019 in PubMed and Embase. Titles and abstracts were evaluated by co-authors for inclusion. Included articles underwent full-text review, data abstraction, and quality assessment. Pooled absolute pretest and posttest risk estimates were calculated for studies evaluating management of patients after treatment.
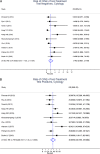
Results: A total of 2,862 articles were identified through the search. Of 50 articles on postcolposcopy, 5 were included for data abstraction. Of 66 articles on posttreatment, 23 were included for data abstraction and were summarized in the meta-analysis. The pooled posttreatment risk of cervical intraepithelial neoplasia (CIN) 2+ in all studies was 4.8% (95% CI = 3.4%-6.8%), ranging from 0.4%-19.5% (τ = 0.57) in individual studies. Among individuals testing negative for human papillomavirus (HPV) posttreatment, the risk of CIN 2+ was 0.69% (95% CI = 0.3%-1.5%); among individuals testing positive for HPV posttreatment, the risk of CIN 2+ was 18.3% (95% CI = 12.1%-26.6%) in all studies. All risk estimates were substantially higher for liquid-based cytology. The HPV-cytology co-testing provided slightly better reassurance compared with HPV alone at the cost of much higher positivity.
Conclusions: Despite a large number of published studies on postcolposcopy and posttreatment surveillance, only few met criteria for abstraction and were included in the meta-analysis. More high-quality studies are needed to evaluate assays and approaches that can improve management of patients with abnormal screening.
Figures



References
-
- Cuschieri K, Ronco G, Lorincz A, et al. Eurogin roadmap 2017: Triage strategies for the management of HPV-positive women in cervical screening programs. Int J Cancer 2018;143:735–45. - PubMed
-
- Wentzensen N, Arbyn M, Berkhof J, et al. Eurogin 2016 Roadmap: how HPV knowledge is changing screening practice. Int J Cancer 2017;140:2192–200. - PubMed
-
- Wentzensen N, Silver MI. Biomarkers for cervical cancer prevention programs: the long and winding road from discovery to clinical use. J Low Genit Tract Dis 2016;20:191–4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

