Prognostic value of carbohydrate antigen125 and carcino embryonic antigen expression in patients with colorectal carcinoma and its guiding significance for chemotherapy
- PMID: 32243362
- PMCID: PMC7220750
- DOI: 10.1097/MD.0000000000019420
Prognostic value of carbohydrate antigen125 and carcino embryonic antigen expression in patients with colorectal carcinoma and its guiding significance for chemotherapy
Abstract
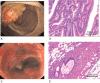
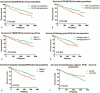
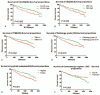
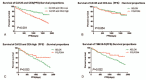
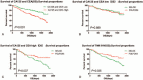
The aim of this study is to evaluate the predictive value of carbohydrate antigen125 (CA125) and carcino embryonic antigen (CEA) expression and its guiding role of choosing chemotherapy regimen in post-operation patients with colorectal carcinoma.The clinical data of all patients, including laboratory data and pathological data, were collected from the electronic medical records. Kaplan-Meier Log rank test, COX regression model and subgroup analyses were employed to assess the correlation between the expression of CA125 and CEA in patients with colorectal carcinoma and the survival, and the effect on chemotherapy efficacy.Kaplan-Meier showed that CA125 expression is negatively related to the progression-free survival (PFS) of the post-operative patients, Median PFS was 1140 days in the patients with high expression, and Median PFS was 1387 days in the patients with low expression (χ = 4.715, P = .030); CEA expression is also negatively associated with the PFS of the post-operative patients, Median PFS was 1197 days in the patients with high expression, and Median PFS was 1424 days in the patients with low expression (χ = 4.992, P = .025). Subgroup analysis also showed that the patients with normal CA125 and CEA had better prognosis, median PFS was 1505 days, and the patients with CA125 and (or) CEA high expression had poor prognosis and median PFS was 1162 days (χ = 13.346, P = .001), and found that there was no statistical difference in patients with oxaliplatin plus capecitabine (XELOX) and oxaliplatin, 5-fluorouracil and Calcium folinate (FOLFOX) chemotherapy in patients with CA125 and CEA low expression. However, in these patients with CA125 or (and) CEA high expression, the median PFS of patients treated with XELOX was 1082 days, and the median PFS of patients treated with FOLFOX chemotherapy was 1335 (χ = 4.547, P = .033).Expression of CA125 and CEA associated with the survival of patients, and have some guiding significance for chemotherapy in patients with colorectal cancer after operation; Compared with XELOX, FOLFOX chemotherapy is more effective for CA125 or (and) CEA high expression patients with colorectal carcinoma.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy.World J Surg Oncol. 2014 Dec 29;12:397. doi: 10.1186/1477-7819-12-397. World J Surg Oncol. 2014. PMID: 25543664 Free PMC article.
-
Bevacizumab with 5-fluorouracil, leucovorin, and oxaliplatin versus bevacizumab with capecitabine and oxaliplatin for metastatic colorectal carcinoma: results of a large registry-based cohort analysis.BMC Cancer. 2014 May 7;14:323. doi: 10.1186/1471-2407-14-323. BMC Cancer. 2014. PMID: 24884897 Free PMC article.
-
Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study.Ann Oncol. 2008 Oct;19(10):1720-6. doi: 10.1093/annonc/mdn370. Epub 2008 Jun 10. Ann Oncol. 2008. PMID: 18550577 Clinical Trial.
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
-
XELOX vs. FOLFOX in metastatic colorectal cancer: An updated meta-analysis.Cancer Invest. 2016;34(2):94-104. doi: 10.3109/07357907.2015.1104689. Epub 2016 Feb 11. Cancer Invest. 2016. PMID: 26864862 Review.
Cited by
-
Prediction models of colorectal cancer prognosis incorporating perioperative longitudinal serum tumor markers: a retrospective longitudinal cohort study.BMC Med. 2023 Feb 21;21(1):63. doi: 10.1186/s12916-023-02773-2. BMC Med. 2023. PMID: 36803500 Free PMC article.
-
Clinical Significance of Early Carcinoembryonic Antigen Change in Patients With Nonmetastatic Colorectal Cancer.Front Oncol. 2022 May 9;12:739614. doi: 10.3389/fonc.2022.739614. eCollection 2022. Front Oncol. 2022. PMID: 35615159 Free PMC article.
-
Metabolomics- and Proteomics-Based Disease Diagnostic Classifier Model for the Prediction and Diagnosis of Colorectal Carcinoma.J Proteome Res. 2025 Apr 4;24(4):2096-2111. doi: 10.1021/acs.jproteome.5c00010. Epub 2025 Mar 19. J Proteome Res. 2025. PMID: 40108892
-
Oxaliplatin combined with capecitabine therapy and comprehensive nursing in advanced colorectal cancer patients.Front Med (Lausanne). 2025 Jul 2;12:1582683. doi: 10.3389/fmed.2025.1582683. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40672837 Free PMC article.
-
Value of TCT combined with serum CA153 and CA50 in early diagnosis of cervical cancer and precancerous lesions.Pak J Med Sci. 2022 Jul-Aug;38(6):1471-1476. doi: 10.12669/pjms.38.6.5503. Pak J Med Sci. 2022. PMID: 35991263 Free PMC article.
References
-
- Wang XF, Wu YH, Wang MS, et al. CEA, AFP, CA125, CA153 and CA199 in malignant pleural effusions predict the cause. Asian Pac J Cancer Prev 2014;15:363–8. - PubMed
-
- Li Y, Li DJ, Chen J, et al. Application of Joint Detection of AFP, CA19-9, CA125 and CEA in Identification and Diagnosis of Cholangiocarcinoma. Asian Pac J Cancer Prev 2015;16:3451–5. - PubMed
-
- Shomaf M, Yousef AL, Ababna N, et al. Cyclooxygenase-2 (COX2) gene polymorphisms and the risk of sporadic colorectal cancer and polyps among Jordanian population. Turk J Gastroenterol 2015;26:154–8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

