Co-culture of induced pluripotent stem cells with cardiomyocytes is sufficient to promote their differentiation into cardiomyocytes
- PMID: 32243463
- PMCID: PMC7122760
- DOI: 10.1371/journal.pone.0230966
Co-culture of induced pluripotent stem cells with cardiomyocytes is sufficient to promote their differentiation into cardiomyocytes
Abstract
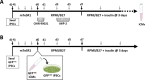
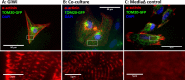
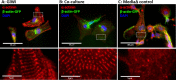
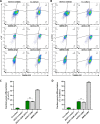
Various types of stem cells and non-stem cells have been shown to differentiate or transdifferentiate into cardiomyocytes by way of co-culture with appropriate inducer cells. However, there is a limited demonstration of a co-culture induction system utilizing stem cell-derived cardiomyocytes as a stimulatory source for cardiac reprogramming (of stem cells or otherwise). In this study, we utilized an inductive co-culture method to show that previously differentiated induced pluripotent stem (iPS) cell-derived cardiomyocytes (iCMs), when co-cultivated with iPS cells, constituted a sufficient stimulatory system to induce cardiac differentiation. To enable tracking of both cell populations, we utilized GFP-labeled iPS cells and non-labeled iCMs pre-differentiated using inhibitors of GSK and Wnt signaling. Successful differentiation was assessed by the exhibition of spontaneous self-contractions, structural organization of α-actinin labeled sarcomeres, and expression of cardiac specific markers cTnT and α-actinin. We found that iCM-iPS cell-cell contact was essential for inductive differentiation, and this required overlaying already adherent iPS cells with iCMs. Importantly, this process was achieved without the exogenous addition of pathway inhibitors and morphogens, suggesting that 'older' iCMs serve as an adequate stimulatory source capable of recapitulating the necessary culture environment for cardiac differentiation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

