Differentially poised vesicles underlie fast and slow components of release at single synapses
- PMID: 32243497
- PMCID: PMC7201884
- DOI: 10.1085/jgp.201912523
Differentially poised vesicles underlie fast and slow components of release at single synapses
Abstract
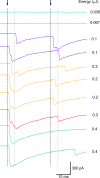
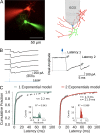
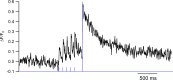
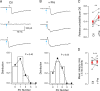
In several types of central mammalian synapses, sustained presynaptic stimulation leads to a sequence of two components of synaptic vesicle release, reflecting the consecutive contributions of a fast-releasing pool (FRP) and of a slow-releasing pool (SRP). Previous work has shown that following common depletion by a strong stimulation, FRP and SRP recover with different kinetics. However, it has remained unclear whether any manipulation could lead to a selective enhancement of either FRP or SRP. To address this question, we have performed local presynaptic calcium uncaging in single presynaptic varicosities of cerebellar interneurons. These varicosities typically form "simple synapses" onto postsynaptic interneurons, involving several (one to six) docking/release sites within a single active zone. We find that strong uncaging laser pulses elicit two phases of release with time constants of ∼1 ms (FRP release) and ∼20 ms (SRP release). When uncaging was preceded by action potential-evoked vesicular release, the extent of SRP release was specifically enhanced. We interpret this effect as reflecting an increased likelihood of two-step release (docking then release) following the elimination of docked synaptic vesicles by action potential-evoked release. In contrast, a subthreshold laser-evoked calcium elevation in the presynaptic varicosity resulted in an enhancement of the FRP release. We interpret this latter effect as reflecting an increased probability of occupancy of docking sites following subthreshold calcium increase. In conclusion, both fast and slow components of release can be specifically enhanced by certain presynaptic manipulations. Our results have implications for the mechanism of docking site replenishment and the regulation of synaptic responses, in particular following activation of ionotropic presynaptic receptors.
© 2020 Blanchard et al.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

