Effects of Prenatal Exposure to a Mixture of Organophosphate Flame Retardants on Placental Gene Expression and Serotonergic Innervation in the Fetal Rat Brain
- PMID: 32243540
- PMCID: PMC7357193
- DOI: 10.1093/toxsci/kfaa046
Effects of Prenatal Exposure to a Mixture of Organophosphate Flame Retardants on Placental Gene Expression and Serotonergic Innervation in the Fetal Rat Brain
Abstract
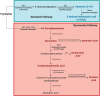
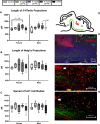
There is a growing need to understand the potential neurotoxicity of organophosphate flame retardants (OPFRs) and plasticizers because use and, consequently, human exposure, is rapidly expanding. We have previously shown in rats that developmental exposure to the commercial flame retardant mixture Firemaster 550 (FM 550), which contains OPFRs, results in sex-specific behavioral effects, and identified the placenta as a potential target of toxicity. The placenta is a critical coordinator of fetal growth and neurodevelopment, and a source of neurotransmitters for the developing brain. We have shown in rats and humans that flame retardants accumulate in placental tissue, and induce functional changes, including altered neurotransmitter production. Here, we sought to establish if OPFRs (triphenyl phosphate and a mixture of isopropylated triarylphosphate isomers) alter placental function and fetal forebrain development, with disruption of tryptophan metabolism as a primary pathway of interest. Wistar rat dams were orally exposed to OPFRs (0, 500, 1000, or 2000 μg/day) or a serotonin (5-HT) agonist 5-methoxytryptamine for 14 days during gestation and placenta and fetal forebrain tissues collected for analysis by transcriptomics and metabolomics. Relative abundance of genes responsible for the transport and synthesis of placental 5-HT were disrupted, and multiple neuroactive metabolites in the 5-HT and kynurenine metabolic pathways were upregulated. In addition, 5-HTergic projections were significantly longer in the fetal forebrains of exposed males. These findings suggest that OPFRs have the potential to impact the 5-HTergic system in the fetal forebrain by disrupting placental tryptophan metabolism.
Keywords: developmental; developmental toxicity; developmental/teratology; endocrine disruptors; endocrine toxicology; flame retardants; metabolome; neurotoxicity; neurotoxicology; neurotransmitter; prenatal; reproductive and developmental toxicology.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures







References
-
- Abdallah M. A., Covaci A. (2014). Organophosphate flame retardants in indoor dust from Egypt: Implications for human exposure. Environ. Sci. Technol. 48, 4782–4789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

