Different Strategies to Execute Multi-Database Studies for Medicines Surveillance in Real-World Setting: A Reflection on the European Model
- PMID: 32243569
- PMCID: PMC7484985
- DOI: 10.1002/cpt.1833
Different Strategies to Execute Multi-Database Studies for Medicines Surveillance in Real-World Setting: A Reflection on the European Model
Abstract
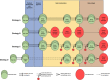
Although postmarketing studies conducted in population-based databases often contain information on patients in the order of millions, they can still be underpowered if outcomes or exposure of interest is rare, or the interest is in subgroup effects. Combining several databases might provide the statistical power needed. A multi-database study (MDS) uses at least two healthcare databases, which are not linked with each other at an individual person level, with analyses carried out in parallel across each database applying a common study protocol. Although many MDSs have been performed in Europe in the past 10 years, there is a lack of clarity on the peculiarities and implications of the existing strategies to conduct them. In this review, we identify four strategies to execute MDSs, classified according to specific choices in the execution: (A) local analyses, where data are extracted and analyzed locally, with programs developed by each site; (B) sharing of raw data, where raw data are locally extracted and transferred without analysis to a central partner, where all the data are pooled and analyzed; (C) use of a common data model with study-specific data, where study-specific data are locally extracted, loaded into a common data model, and processed locally with centrally developed programs; and (D) use of general common data model, where all local data are extracted and loaded into a common data model, prior to and independent of any study protocol, and protocols are incorporated in centrally developed programs that run locally. We illustrate differences between strategies and analyze potential implications.
© 2020 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
R.G.’s institution participates in multi‐database studies, which have adopted each of the strategies described in this paper. Some studies are funded by the Innovative Medicines Initiative, Novartis, Eli Lilly, and Daiichi Sankyo, and all are compliant with the ENCePP Code of Conduct for transparency and scientific independence. M.C.J.S. is coordinating and has coordinated/led several EU and global publicly funded projects developing and using common data models to assess medication safety in multiple data sources, these include IMI‐Conception, IMI‐ADVANCE, FP7‐GRIP, FP7‐SOS, FP7‐ARITMO, FP7‐SAFEGUARD, ECDC‐VAESCO, CDC‐SOMNIA, FP‐7 EU‐ADR, and FP‐6 TEDDY. She is/was also principal investigator in several multi‐database postauthorization safety studies required or commissioned by the EMA, sponsored by Novartis, Servier, and GSK, and all are compliant with the ENCePP Code of Conduct for transparency and scientific independence. G.R. works as scientific consultant for Agenzia regionale di sanità della Toscana, which participates in different multi‐database studies. Some studies are funded by the Innovative Medicines Initiative, Novartis, Eli Lilly, and Daiichi Sankyo, and all are compliant with the ENCePP Code of Conduct for transparency and scientific independence. T.S. is working at an independent, nonprofit research institute, the Leibniz Institute for Prevention Research and Epidemiology—BIPS. Unrelated to this paper, BIPS occasionally conducts studies financed by the pharmaceutical industry. Almost exclusively, these are post‐authorization safety studies requested by health authorities. All studies are compliant with the ENCePP Code of Conduct for transparency and scientific independence. G.T. coordinates an academic team at University of Messina that received in the last 2 years unconditional grants for the conduct of observational studies from Daiichi Sankyo, AstraZeneca, PTC Therapeutics, and Amgen. All other authors declared no competing interests for this work.
Figures
References
-
- Skovlund, E. , Leufkens, H.G.M. & Smyth, J.F. The use of real‐world data in cancer drug development. Eur. J. Cancer 101, 69–76 (2018). - PubMed
-
- Jarow, J.P. , LaVange, L. & Woodcock, J. Multidimensional evidence generation and FDA regulatory decision making. Defining and using "real‐world" data. JAMA 318, 703–704 (2017). - PubMed
-
- Pitts, P.J. , Louet, H.L. , Moride, Y. & Conti, R.M. 21st century pharmacovigilance: efforts, roles, and responsibilities. Lancet Oncol. 17, e486–e492 (2016). - PubMed
-
- Klungel, O.H. et al. Multi‐centre, multi‐database studies with common protocols: lessons learnt from the IMI PROTECT project. Pharmacoepidemiol. Drug Saf. 25 (Suppl. 1), 156–165 (2016). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

