The benefits of iron supplementation following blood donation vary with baseline iron status
- PMID: 32243609
- PMCID: PMC7393577
- DOI: 10.1002/ajh.25800
The benefits of iron supplementation following blood donation vary with baseline iron status
Abstract
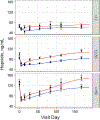
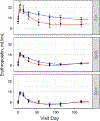
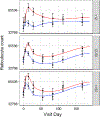
Whole blood donation rapidly removes approximately 10% of a donor's blood volume and stimulates substantial changes in iron metabolism and erythropoiesis. We sought to identify donors who benefit from iron supplementation, describe the nature of the benefit, and define the time course for recovery from donation. Blood samples were collected over 24 weeks following whole blood donation from 193 participants, with 96 participants randomized to 37.5 mg daily oral iron. Changes in total body, red blood cell (RBC), and storage iron, hepcidin, erythropoietin, and reticulocyte count were modeled using semiparametric curves in a mixed model. and the changes were compared among six groups defined by baseline ferritin (<12; 12-50; ≥50 ng/mL) and iron supplementation. The effect of oral iron on storage and RBC iron recovery was minimal in donors with baseline ferritin ≥50 ng/mL, but sizeable when ferritin was <50 ng/mL. Iron initially absorbed went to RBC and storage iron pools when ferritin was <12 ng/mL but went mostly to RBCs when ferritin was ≥12 ng/mL. Donors with ferritin ≥12 ng/mL had a "ripple" increase in reticulocytes ~100 days after donation indicating physiological responses occur months following donation. Thus, iron supplements markedly enhance recovery from whole blood donation in donors with ferritin <50 ng/mL. However, full recovery from donation requires over 100 days when taking iron. The findings also highlight the value of the study of blood donors for understanding human hemoglobin and iron metabolism and their usefulness for future studies as additional biomarkers are discovered.
Trial registration: ClinicalTrials.gov NCT01555060.
© 2020 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
B.R.S. serves on the advisory board of HemaStrat. AEM receives research grant funding from Novo Nordisk and has received honoraria from Novo Nordisk for serving on Advisory Boards. The other authors have no competing interests.
Figures
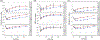
References
-
- Schotten N, Pasker-de Jong PC, Moretti D, et al. The donation interval of 56 days requires extension to 180 days for whole blood donors to recover from changes in iron metabolism. Blood. 2016;128:2185–2188. - PubMed
-
- Mast AE, Foster TM, Pinder HL, et al. Behavioral, biochemical, and genetic analysis of iron metabolism in high-intensity blood donors. Transfusion. 2008;48:2197–2204. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
