The Emerging Landscape of Immune Cell Therapies
- PMID: 32243795
- PMCID: PMC8900215
- DOI: 10.1016/j.cell.2020.03.001
The Emerging Landscape of Immune Cell Therapies
Abstract
Cell therapies present an entirely new paradigm in drug development. Within this class, immune cell therapies are among the most advanced, having already demonstrated definitive evidence of clinical benefits in cancer and infectious disease. Numerous features distinguish these "living therapies" from traditional medicines, including their ability to expand and contract in proportion to need and to mediate therapeutic benefits for months or years following a single application. Continued advances in fundamental immunology, genetic engineering, gene editing, and synthetic biology exponentially expand opportunities to enhance the sophistication of immune cell therapies, increasing potency and safety and broadening their potential for treatment of disease. This perspective will summarize the current status of immune cell therapies for cancer, infectious disease, and autoimmunity, and discuss advances in cellular engineering to overcome barriers to progress.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests C.L.M. is an inventor on numerous patent applications in the area of CAR T cell immunotherapy and has received royalties for the CD22-CAR from the NIH following licensure to Opus Bio and Juno Therapeutics. C.L.M. is a founder of, holds equity in, and receives consulting fees from Lyell Immunopharma, which develops cellular therapies for cancer. She is also a consultant for Nektar, Neoimmune Tech, and Apricity and holds equity in Apricity and Allogene. M.V.M. is an inventor on numerous patent applications in the area of CAR T cell immunotherapy and has received royalties. M.V.M. is a consultant or advisory board member for multiple companies developing cellular therapies and holds equity in TCR2 and Century therapeutics. E.W.W. is an inventor on numerous patent applications in the area of CAR T cell immunotherapy and holds equity in and receives consulting fees from Lyell Immunopharma.
Figures
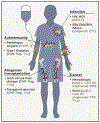

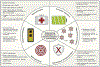
References
-
- Abramson JS, Palomba ML, Gordon LI, Lunning MA, Arnason JE, Wang M, Forero A, Maloney DG, Albertson T, Garcia J, et al. (2017). High Durable CR Rates in Relapsed/Refractory (R/R) Aggressive B-NHL Treated with the CD19-Directed CAR T Cell Product JCAR017 (TRANSCEND NHL 001): Defined Composition Allows for Dose-Finding and Definition of Pivotal Cohort. Blood 130, 581–581.
-
- Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, et al. (2015). Human Epidermal Growth Factor Receptor 2 (HER2) - Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J Clin Oncol 33, 1688–1696. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

