Developing Organoids from Ovarian Cancer as Experimental and Preclinical Models
- PMID: 32243841
- PMCID: PMC7160357
- DOI: 10.1016/j.stemcr.2020.03.004
Developing Organoids from Ovarian Cancer as Experimental and Preclinical Models
Abstract
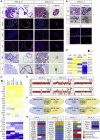
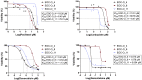
Ovarian cancer (OC) represents the most dismal gynecological cancer. Pathobiology is poorly understood, mainly due to lack of appropriate study models. Organoids, defined as self-developing three-dimensional in vitro reconstructions of tissues, provide powerful tools to model human diseases. Here, we established organoid cultures from patient-derived OC, in particular from the most prevalent high-grade serous OC (HGSOC). Testing multiple culture medium components identified neuregulin-1 (NRG1) as key factor in maximizing OC organoid development and growth, although overall derivation efficiency remained moderate (36% for HGSOC patients, 44% for all patients together). Established organoid lines showed patient tumor-dependent morphology and disease characteristics, and recapitulated the parent tumor's marker expression and mutational landscape. Moreover, the organoids displayed tumor-specific sensitivity to clinical HGSOC chemotherapeutic drugs. Patient-derived OC organoids provide powerful tools for the study of the cancer's pathobiology (such as importance of the NRG1/ERBB pathway) as well as advanced preclinical tools for (personalized) drug screening and discovery.
Keywords: ERBB; disease modeling; high-grade serous ovarian cancer; neuregulin-1; organoids; ovarian cancer.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Antoniou A., Pharoah P.D.P., Narod S., Risch H.A., Eyfjord J.E., Hopper J.L., Loman N., Olsson H., Johannsson O., Borg Å. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am. J. Hum. Genet. 2003;72:1117–1130. - PMC - PubMed
-
- Aune G., Lian A.M., Tingulstad S., Torp S.H., Forsmo S., Reseland J.E., Stunes A.K., Syversen U. Increased circulating hepatocyte growth factor (HGF): a marker of epithelial ovarian cancer and an indicator of poor prognosis. Gynecol. Oncol. 2011;121:402–406. - PubMed
-
- Azmi A.S., Li Y., Muqbil I., Aboukameel A., Senapedis W., Baloglu E., Landesman Y., Shacham S., Kauffman M.G., Philip P.A. Exportin 1 (XPO1) inhibition leads to restoration of tumor suppressor miR-145 and consequent suppression of pancreatic cancer cell proliferation and migration. Oncotarget. 2017;8:82144–82155. - PMC - PubMed
-
- Boretto M., Cox B., Noben M., Hendriks N., Fassbender A., Roose H., Amant F., Timmerman D., Tomassetti C., Vanhie A. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 2017;144:1775–1786. - PubMed
-
- Boretto M., Maenhoudt N., Luo X., Hennes A., Boeckx B.B., Bui B., Heremans R., Perneel L., Kobayashi H., Van Zundert I. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019;21:1041–1051. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

